
Draw the structures of following compounds:
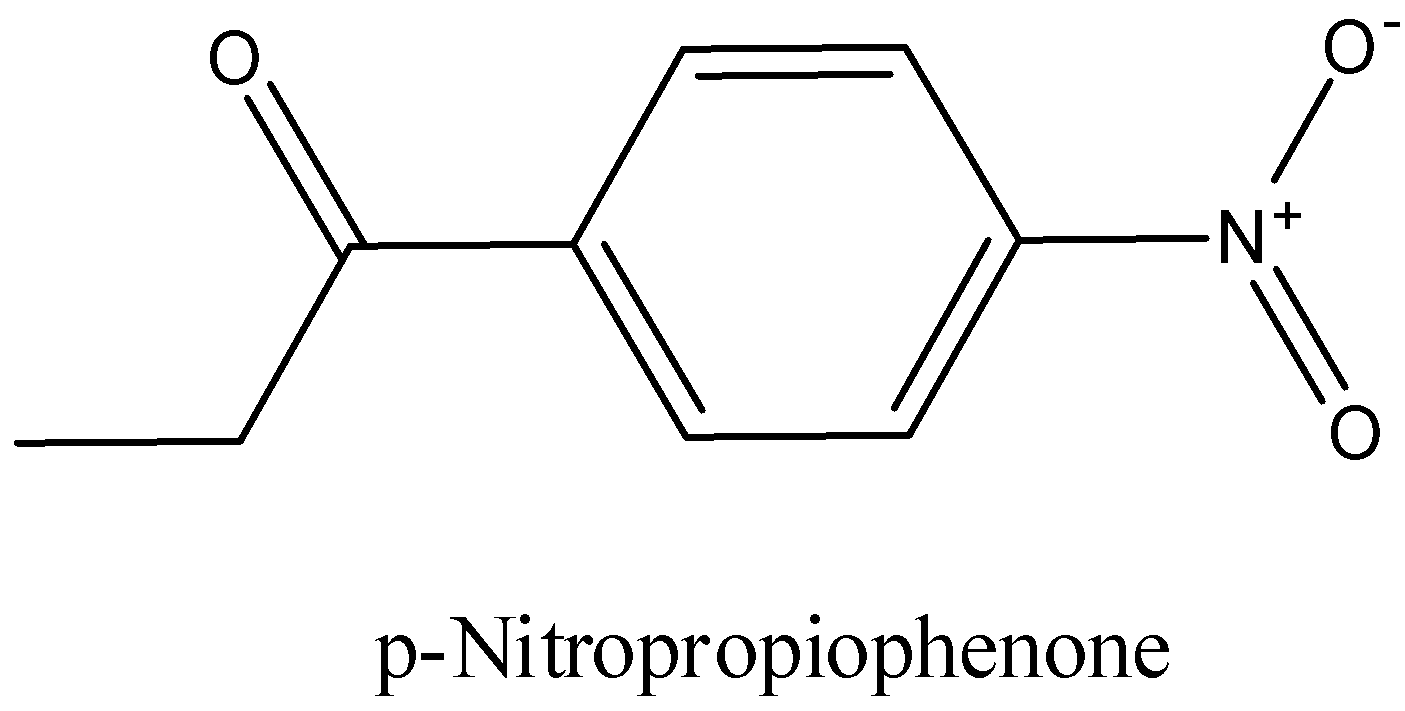
p-Nitropropiophenone
Answer
502.2k+ views
Hint: We have to know that the IUPAC framework is the most reasonable and broadly utilized arrangement of classification in natural science. The IUPAC of any natural compound comprises three sections in particular, word root, postfix (optional or essential) and prefix (auxiliary or essential).
Complete answer:
We have to know that the word root prop recommends that there are three carbon molecules in the parent chain. Which contains the practical gathering ketone from the outset position, the postfix shows that the utilitarian gathering ketone is likewise joined to phenyl bunch and the prefix demonstrates that a nitro bunch is available at para position to the useful gathering.
Therefore, the structure of p-Nitropropiophenone is drawn below,
Additional information:
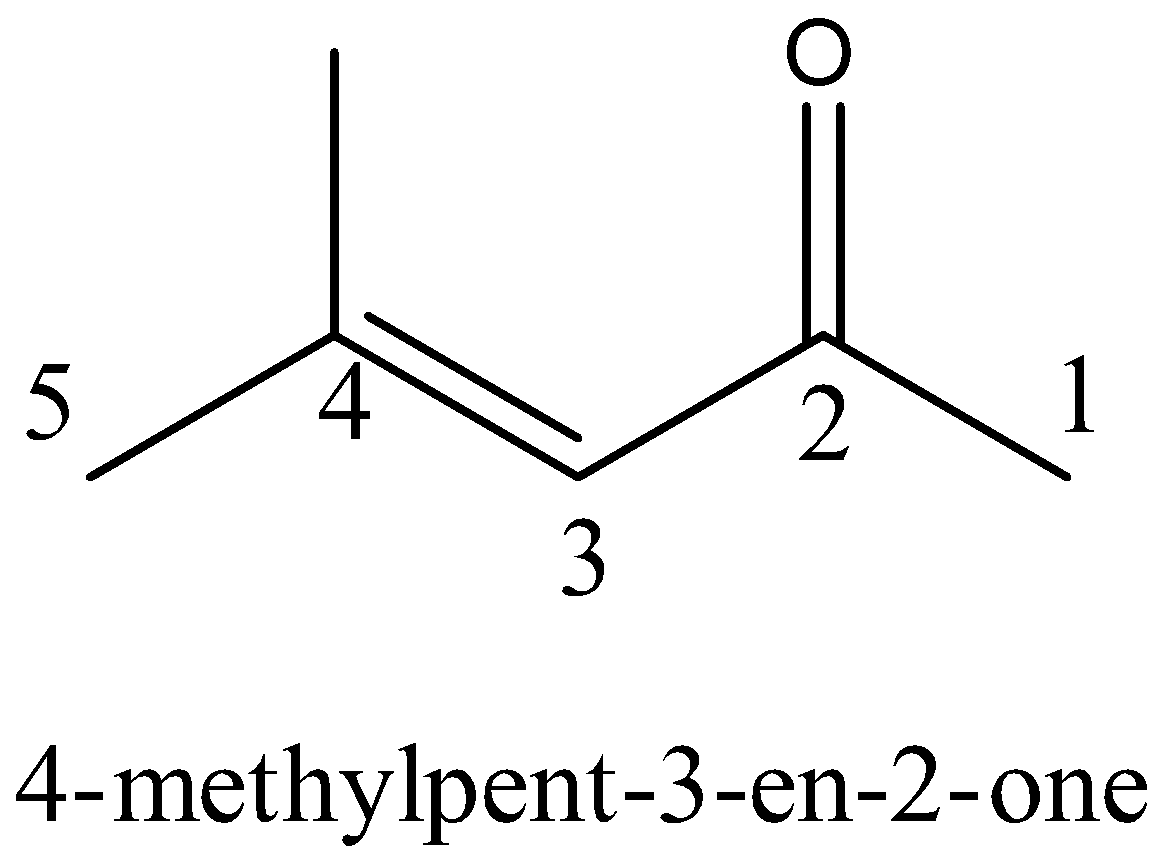
For $4 - methylpent - 3 - en - 2 - one$ ,
The word root confined recommends that there are five carbon particles in the parent chain, the prefix shows that a methyl bunch is subbed at fourth position. The essential postfix en shows that the parent chain is unsaturated and there is a twofold security (alkene) among third and fourth carbon particles. The optional additional one demonstrates that a ketone is present at the second situation of carbon.
Note:
In the event that two chains of equivalent lengths are conceivable, the one with the bigger number of side chains will be the parent chain. The parent chain is numbered so that the utilitarian gathering gets the least vacant. At the point when an enormous and complex gathering is joined to a benzene ring it is entirely expected to name the particle as an alkane, alkene and benzene as side chain subordinate phenyl.
Complete answer:
We have to know that the word root prop recommends that there are three carbon molecules in the parent chain. Which contains the practical gathering ketone from the outset position, the postfix shows that the utilitarian gathering ketone is likewise joined to phenyl bunch and the prefix demonstrates that a nitro bunch is available at para position to the useful gathering.
Therefore, the structure of p-Nitropropiophenone is drawn below,

Additional information:
For $4 - methylpent - 3 - en - 2 - one$ ,
The word root confined recommends that there are five carbon particles in the parent chain, the prefix shows that a methyl bunch is subbed at fourth position. The essential postfix en shows that the parent chain is unsaturated and there is a twofold security (alkene) among third and fourth carbon particles. The optional additional one demonstrates that a ketone is present at the second situation of carbon.

Note:
In the event that two chains of equivalent lengths are conceivable, the one with the bigger number of side chains will be the parent chain. The parent chain is numbered so that the utilitarian gathering gets the least vacant. At the point when an enormous and complex gathering is joined to a benzene ring it is entirely expected to name the particle as an alkane, alkene and benzene as side chain subordinate phenyl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




