
Draw the structure of the silicate anion in:
(a) ${K_4}Si{O_4}$
(b) $A{g_{10}}S{i_4}{O_{13}}$
Answer
543.9k+ views
Hint: Silicate is the member of a group of anions which consists of silicon and oxygen. The general formula of silicates is ${\left[ {Si{O_{4 - x}}^{\left( {4 - 2x} \right) - }} \right]_n}$ where x can be 0 or greater than 0 and less than 2. The lowest form of silicates is $Si{O_4}^{4 - }$.
Complete step-by-step answer:
In the compound ${K_4}Si{O_4}$ the anion part is $Si{O_4}^{4 - }$. It is called orthosilicate. Orthosilicate is sometimes called silicon tetroxide because there is one Si and four oxygen surrounds it. Silicates of potassium are manufactured in ordinary furnaces of glass. The uses of potassium orthosilicate are that it is used as adhesive and sealant chemical. It can also be used as agricultural chemical, plating and surface treating agents. For industrial and commercial use application, it is a hard surface cleaner. The structure of orthosilicate or $Si{O_4}^{4 - }$ is as follows,
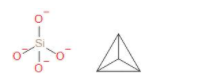
In the compound $A{g_{10}}S{i_4}{O_{13}}$ the anion part is $S{i_4}{O_{13}}^{10 - }$. $A{g_{10}}S{i_4}{O_{13}}$ exhibits excellent photocatalytic activity towards degradation of organic molecules. The structure of $S{i_4}{O_{13}}^{10 - }$ is as follows,
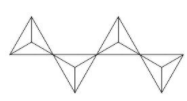
Here in this compound, oxygen attached is shared between two Si atoms.
Note: Solid silicates are usually stable and have been characterized well. Silicates with alkali cations are partially soluble in water. Silicate can be natural and artificial and they can be used for artistic activity. The most abundant mineral groups on earth are silicates. They have many technological uses too. The quartz crystals used in watches and radios are also silicates which can produce rhythmic high frequency vibration. To protect from extreme temperatures and outer atmosphere, silicate ceramic tiles possessing thermal properties are used on space shuttles.
Complete step-by-step answer:
In the compound ${K_4}Si{O_4}$ the anion part is $Si{O_4}^{4 - }$. It is called orthosilicate. Orthosilicate is sometimes called silicon tetroxide because there is one Si and four oxygen surrounds it. Silicates of potassium are manufactured in ordinary furnaces of glass. The uses of potassium orthosilicate are that it is used as adhesive and sealant chemical. It can also be used as agricultural chemical, plating and surface treating agents. For industrial and commercial use application, it is a hard surface cleaner. The structure of orthosilicate or $Si{O_4}^{4 - }$ is as follows,
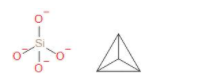
In the compound $A{g_{10}}S{i_4}{O_{13}}$ the anion part is $S{i_4}{O_{13}}^{10 - }$. $A{g_{10}}S{i_4}{O_{13}}$ exhibits excellent photocatalytic activity towards degradation of organic molecules. The structure of $S{i_4}{O_{13}}^{10 - }$ is as follows,

Here in this compound, oxygen attached is shared between two Si atoms.
Note: Solid silicates are usually stable and have been characterized well. Silicates with alkali cations are partially soluble in water. Silicate can be natural and artificial and they can be used for artistic activity. The most abundant mineral groups on earth are silicates. They have many technological uses too. The quartz crystals used in watches and radios are also silicates which can produce rhythmic high frequency vibration. To protect from extreme temperatures and outer atmosphere, silicate ceramic tiles possessing thermal properties are used on space shuttles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




