
Draw the structure of the following molecule:
\[Br{{F}_{5}}\]
Answer
576.9k+ views
Hint:. Identify the compound given in the question with their common name/IUPAC name. Now identify the central atom in each compound and calculate the oxidation number for the same atom as well. With the help of oxidation state, you can determine the number of bonds that can be made around the atom and finally draw the complete expanded structure of the compound.
Complete step by step answer:
The name of the compound is bromine pentafluoride. It is described as an interhalogen compound as well as a strong fluorinating agent.
-It is commonly used in the analysis of oxygen isotope. It is also in the testing phase for the role of oxidizer in liquid rocket propellants as well as the fluorinating agent in the processing of uranium.
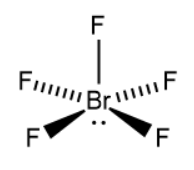
-We will now try to draw the structure of bromine pentafluoride. The central atom in the compound can be identified as bromine.
-Since 5 fluorine atoms are attached to the bromine atom, the oxidation state of bromine in bromine pentafluoride is +5.
-Along with 5 bonds, the central atom has 1 lone pair of electrons.
Based on the above information, the possible structure of \[Br{{F}_{5}}\] becomes,
Note: Bromine and fluorine belong to the 17th group of the periodic table. The elements of the halogen group are considered to be the most electronegative element in their respective period. However, the electronegativity character decreases down the group. This is the reason why bromine has positive oxidation state and fluorine has negative oxidation state of +5 and -1 respectively.
Complete step by step answer:
The name of the compound is bromine pentafluoride. It is described as an interhalogen compound as well as a strong fluorinating agent.
-It is commonly used in the analysis of oxygen isotope. It is also in the testing phase for the role of oxidizer in liquid rocket propellants as well as the fluorinating agent in the processing of uranium.
-We will now try to draw the structure of bromine pentafluoride. The central atom in the compound can be identified as bromine.
-Since 5 fluorine atoms are attached to the bromine atom, the oxidation state of bromine in bromine pentafluoride is +5.
-Along with 5 bonds, the central atom has 1 lone pair of electrons.
Based on the above information, the possible structure of \[Br{{F}_{5}}\] becomes,

Note: Bromine and fluorine belong to the 17th group of the periodic table. The elements of the halogen group are considered to be the most electronegative element in their respective period. However, the electronegativity character decreases down the group. This is the reason why bromine has positive oxidation state and fluorine has negative oxidation state of +5 and -1 respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




