
Draw the structure of the following:
( i ) \[{\rm{Xe}}{{\rm{O}}_{\rm{3}}}\]
( ii ) \[{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
Answer
592.5k+ views
Hint: For hybridisation determination, use the following formula:
\[{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on sulphur }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\]
Complete step by step answer:
( i ) \[{\rm{Xe}}{{\rm{O}}_{\rm{3}}}\]
The central xenon atom has 8 valence electrons. The molecule is electrically neutral with 0 overall charge. Determine the type of the hybridisation:
\[\begin{array}{l}
{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on xenon }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\\
{\rm{Hybridisation = }}\dfrac{{8 + 0 - 0}}{2}\\
{\rm{Hybridisation = }}\dfrac{8}{2}\\
{\rm{Hybridisation = }}4{\rm{ }}
\end{array}\]
Here the number of monovalent groups is taken as zero as oxygen is a bivalent group.
Hybridisation =4 suggests \[{\rm{s}}{{\rm{p}}^3}\] hybridisation. Three bonding domains and one lone pair of electrons are present around the central xenon atom. The electron pair geometry is tetrahedral and the molecular geometry is trigonal planar. The structure is as shown below:
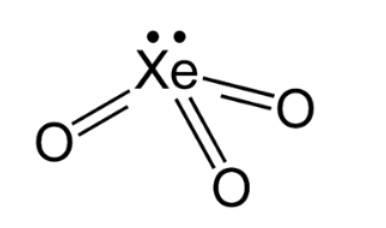
( ii ) \[{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
The central sulphur atom has 6 valence electrons. The molecule is electrically neutral with 0 overall charge. Determine the type of the hybridisation:
\[\begin{array}{l}
{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on sulphur }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\\
{\rm{Hybridisation = }}\dfrac{{6 + 2 - 0}}{2}\\
{\rm{Hybridisation = }}\dfrac{8}{2}\\
{\rm{Hybridisation = }}4{\rm{ }}
\end{array}\]
Here the number of monovalent groups is taken as two as two hydroxyl groups are monovalent groups and oxygen (having double bond) is bivalent group.
Hybridisation =4 suggests \[{\rm{s}}{{\rm{p}}^3}\] hybridisation. Four bonding domains and zero lone pairs of electrons are present around the central sulphur atom. The geometry is tetrahedral. The structure is as shown below:
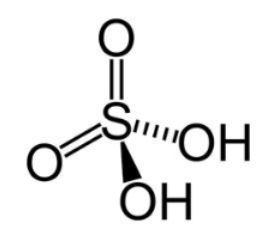
Note:
Hybridisation is the process of mixing two or more atomic orbitals, having almost the same energy, to form the same number of identical and degenerate new types of orbitals. The new orbitals formed are called hybrid orbitals. Hybridisation is used in organic and inorganic chemistry. Hybridisation is a simple theoretical model used to explain molecular geometry. The theory of hybridisation was needed as the original valence bond theory could not explain the molecular geometry.
\[{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on sulphur }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\]
Complete step by step answer:
( i ) \[{\rm{Xe}}{{\rm{O}}_{\rm{3}}}\]
The central xenon atom has 8 valence electrons. The molecule is electrically neutral with 0 overall charge. Determine the type of the hybridisation:
\[\begin{array}{l}
{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on xenon }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\\
{\rm{Hybridisation = }}\dfrac{{8 + 0 - 0}}{2}\\
{\rm{Hybridisation = }}\dfrac{8}{2}\\
{\rm{Hybridisation = }}4{\rm{ }}
\end{array}\]
Here the number of monovalent groups is taken as zero as oxygen is a bivalent group.
Hybridisation =4 suggests \[{\rm{s}}{{\rm{p}}^3}\] hybridisation. Three bonding domains and one lone pair of electrons are present around the central xenon atom. The electron pair geometry is tetrahedral and the molecular geometry is trigonal planar. The structure is as shown below:

( ii ) \[{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\]
The central sulphur atom has 6 valence electrons. The molecule is electrically neutral with 0 overall charge. Determine the type of the hybridisation:
\[\begin{array}{l}
{\rm{Hybridisation = }}\dfrac{{{\rm{ }}\begin{array}{*{20}{c}}
{{\rm{ Number of valence electrons }}}\\
{{\rm{ on sulphur }}}
\end{array}{\rm{ + }}\begin{array}{*{20}{c}}
{{\rm{number of monovalent }}}\\
{{\rm{groups attached }}}
\end{array} - \begin{array}{*{20}{c}}
{{\rm{ charge }}}\\
{{\rm{ with sign }}}
\end{array}{\rm{ }}}}{2}\\
{\rm{Hybridisation = }}\dfrac{{6 + 2 - 0}}{2}\\
{\rm{Hybridisation = }}\dfrac{8}{2}\\
{\rm{Hybridisation = }}4{\rm{ }}
\end{array}\]
Here the number of monovalent groups is taken as two as two hydroxyl groups are monovalent groups and oxygen (having double bond) is bivalent group.
Hybridisation =4 suggests \[{\rm{s}}{{\rm{p}}^3}\] hybridisation. Four bonding domains and zero lone pairs of electrons are present around the central sulphur atom. The geometry is tetrahedral. The structure is as shown below:

Note:
Hybridisation is the process of mixing two or more atomic orbitals, having almost the same energy, to form the same number of identical and degenerate new types of orbitals. The new orbitals formed are called hybrid orbitals. Hybridisation is used in organic and inorganic chemistry. Hybridisation is a simple theoretical model used to explain molecular geometry. The theory of hybridisation was needed as the original valence bond theory could not explain the molecular geometry.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




