
Draw the structure of the following:
1) \[Xe{F_4}\]
2) \[HCl{O_4}\]
Answer
569.7k+ views
Hint:As we know that chemical structures of compounds determine the molecular geometry of that compound by depicting its bond and atoms arrangement. So, to draw a structure of any compound we should know its elements, valence electrons, lone pairs of electrons as well as bond pairs of electrons.
Complete answer:
1. To draw the correct structure of \[Xe{F_4}\]. We will go step by step as shown below:-
Step 1:- First we will find out the elements present in the compound, we can see that in \[Xe{F_4}\] the elements are Xenon and Fluorine.
Step 2:- Next step is to find out the valence electron of each atom and we can see that Xenon has the $8$ valence electrons in its outermost shell. Similarly fluorine has $7$valence electrons in its outermost shell.
Step 3:- Next step is to find out any lone pair electrons in the compound and we can see that there are two lone pairs and four bond pairs of electrons in xenon tetrafluoride. Hence, \[Xe{F_4}\] has octahedral geometry and the structure can be drawn as shown below:
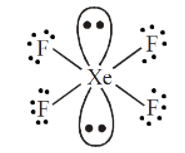
2. To draw the correct structure of \[HCl{O_4}\], we will go step by step as shown below:
Step 1: First we will find out the elements present in the compound, we can see that in \[HCl{O_4}\] the elements are Hydrogen, chlorine and oxygen.
Step 2: Next step is to find the valence electron of each atom and we can see that Hydrogen has the $1$ valence electron in its outermost shell. Similarly chlorine has $7$ valence electrons and oxygen has $6$ valence electrons in its outermost shell.
Step 3: Next step is to find out any lone pair electrons in the compound and we can see that there are eight lone pairs of electrons and the structure can be drawn as shown below:
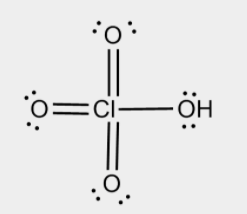
Note:
Xenon tetrafluoride is a strong fluorinating agent and generally obtained by the chemical reaction of xenon with fluorine. It has an octahedral geometry. Perchloric acid is used to produce ammonium perchlorate which is used in rocket fuel. It has a tetrahedral geometry.
Complete answer:
1. To draw the correct structure of \[Xe{F_4}\]. We will go step by step as shown below:-
Step 1:- First we will find out the elements present in the compound, we can see that in \[Xe{F_4}\] the elements are Xenon and Fluorine.
Step 2:- Next step is to find out the valence electron of each atom and we can see that Xenon has the $8$ valence electrons in its outermost shell. Similarly fluorine has $7$valence electrons in its outermost shell.
Step 3:- Next step is to find out any lone pair electrons in the compound and we can see that there are two lone pairs and four bond pairs of electrons in xenon tetrafluoride. Hence, \[Xe{F_4}\] has octahedral geometry and the structure can be drawn as shown below:

2. To draw the correct structure of \[HCl{O_4}\], we will go step by step as shown below:
Step 1: First we will find out the elements present in the compound, we can see that in \[HCl{O_4}\] the elements are Hydrogen, chlorine and oxygen.
Step 2: Next step is to find the valence electron of each atom and we can see that Hydrogen has the $1$ valence electron in its outermost shell. Similarly chlorine has $7$ valence electrons and oxygen has $6$ valence electrons in its outermost shell.
Step 3: Next step is to find out any lone pair electrons in the compound and we can see that there are eight lone pairs of electrons and the structure can be drawn as shown below:

Note:
Xenon tetrafluoride is a strong fluorinating agent and generally obtained by the chemical reaction of xenon with fluorine. It has an octahedral geometry. Perchloric acid is used to produce ammonium perchlorate which is used in rocket fuel. It has a tetrahedral geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




