
Draw the structure of resorcinol and give its IUPAC name.
Answer
560.4k+ views
Hint: The structure of resorcinol includes two hydroxyl groups on the benzene ring. The two hydroxyl groups are separated by one carbon atom.
Complete step by step solution:
The structure of resorcinol is as follow:
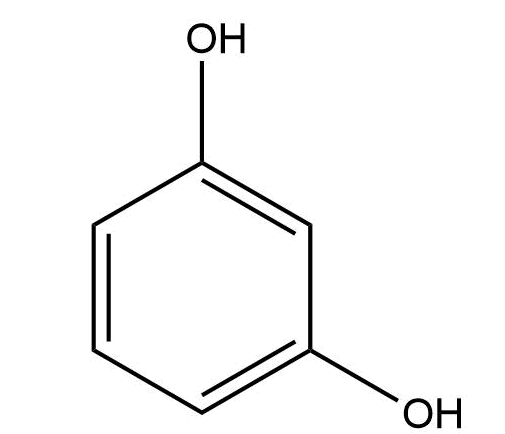
The IUPAC name is Benzene-1,3-diol.
Additional information:
Resorcinol is a white water soluble solid but is insoluble in chloroform and carbon disulphide. Resorcinol is also soluble in alcohol and ether. There are various ways for the production of resorcinol. It is commonly used during the functional group detection in qualitative analysis. It reduces Fehling solution and does not form precipitate with less lead acetate solution. It is also used as a colouring agent during chromatography. When resorcinol is reacted with sodium nitrite it forms a blue dye and is used as an indicator it turns red when present in an acidic solution. Resorcinol undergoes both electrophilic aromatic addition and nucleophilic substitution reaction. It is mainly used in the production of resins and adhesive. It forms adhesive in reaction with the phenol. In medical terms it is used for the acne treatment with less concentration. It requires prescription when used in higher concentrations. It is an active ingredient in the production of resinol, vagisil and Clearasil.
Note: Resorcinol also acts as a chemical intermediate. It is used for the formation of pharmaceuticals and other organic compounds. It is also used in the production of diazo dyes and plasticizers and as a UV absorber in resins. Resorcinol is an analytical reagent and is used for the qualitative determination of ketoses. Resorcinol reacts with formaldehyde to form a thermoset resin which can form the basis of an aerogel.
Complete step by step solution:
The structure of resorcinol is as follow:

The IUPAC name is Benzene-1,3-diol.
Additional information:
Resorcinol is a white water soluble solid but is insoluble in chloroform and carbon disulphide. Resorcinol is also soluble in alcohol and ether. There are various ways for the production of resorcinol. It is commonly used during the functional group detection in qualitative analysis. It reduces Fehling solution and does not form precipitate with less lead acetate solution. It is also used as a colouring agent during chromatography. When resorcinol is reacted with sodium nitrite it forms a blue dye and is used as an indicator it turns red when present in an acidic solution. Resorcinol undergoes both electrophilic aromatic addition and nucleophilic substitution reaction. It is mainly used in the production of resins and adhesive. It forms adhesive in reaction with the phenol. In medical terms it is used for the acne treatment with less concentration. It requires prescription when used in higher concentrations. It is an active ingredient in the production of resinol, vagisil and Clearasil.
Note: Resorcinol also acts as a chemical intermediate. It is used for the formation of pharmaceuticals and other organic compounds. It is also used in the production of diazo dyes and plasticizers and as a UV absorber in resins. Resorcinol is an analytical reagent and is used for the qualitative determination of ketoses. Resorcinol reacts with formaldehyde to form a thermoset resin which can form the basis of an aerogel.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




