
Draw the structure of 3-Bromopentane is:
(a)- $C{{H}_{3}}-C{{H}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}$
(b)- $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}(Br)$
(c)- Both a and b
(d)- None of the above
Answer
584.7k+ views
Hint: The word penta means 5, so pentane must be having five carbon atoms present. In pentane the number of carbon atoms is 5 and the number of hydrogen atoms is 12. 3-Bromoprentane means the 3rd carbon atom contains the bromine substituent.
Complete step by step answer:
Pentane is the member of the homologous group called alkane. The general formula of alkanes is expressed as ${{C}_{n}}{{H}_{2n+2}}$.
The word penta means 5, so pentane must be having five carbon atoms present and pentane means, five carbon atoms in the alkane group.
When we put n as 5 in the general formula of alkane, we get:
${{C}_{5}}{{H}_{12}}$.
The structure of pentane is given below:
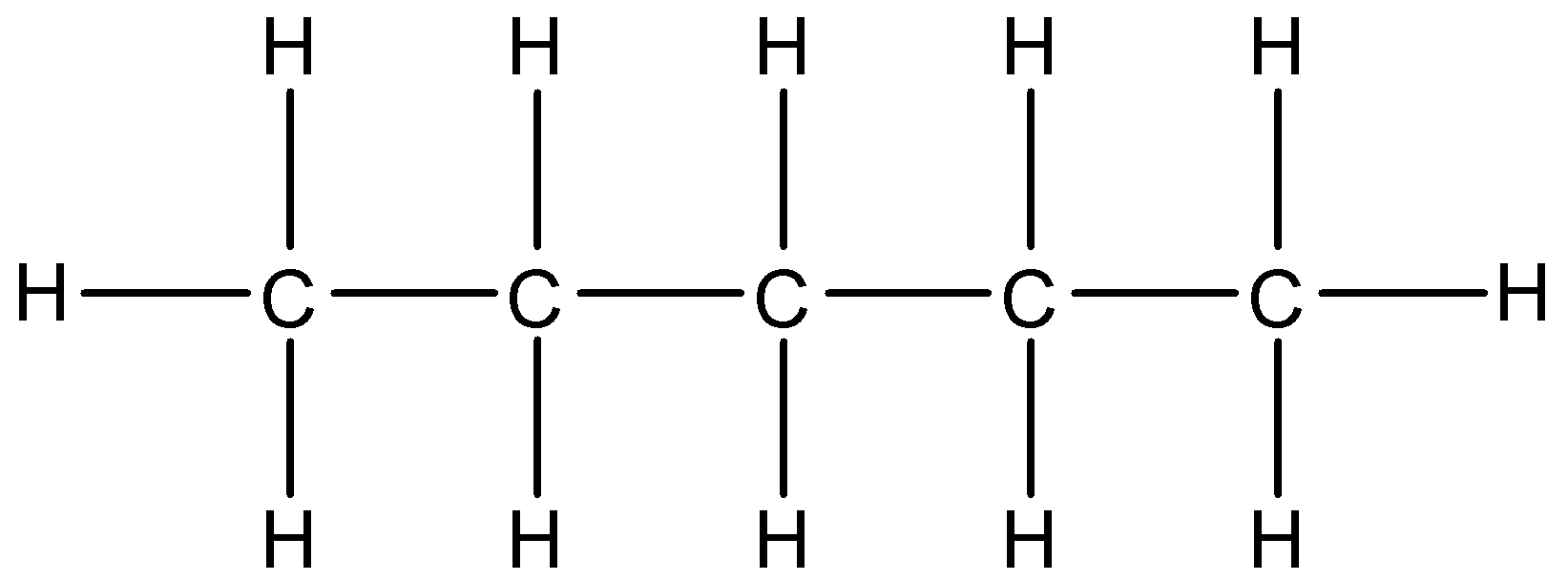
3-Bromopentane means the 3rd carbon atom must be having the bromine substituent. The bromine is attached to the carbon atom by substituting one hydrogen atom present at the 3rd carbon atom. The formula of 3-Bromopentane is\[{{C}_{5}}{{H}_{11}}Br\]. The expanded structure of 3-Bromopentane is given below:
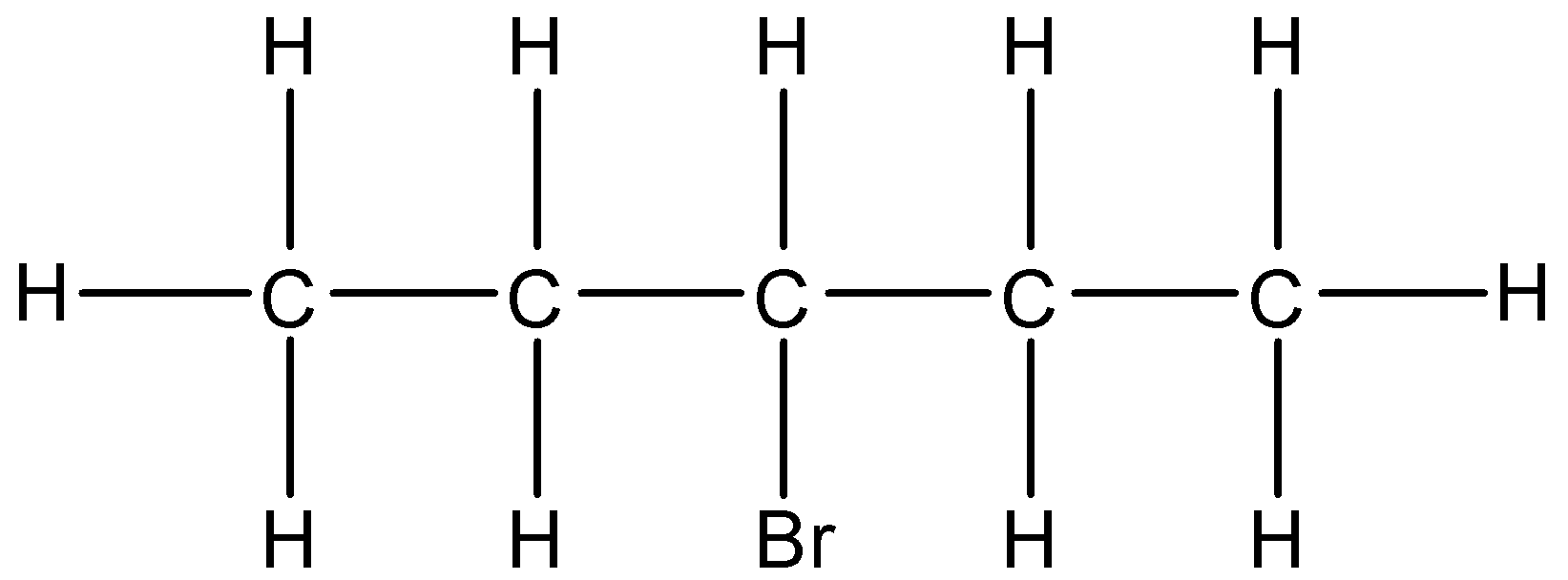
So, in the condensed form we can write $C{{H}_{3}}-C{{H}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}$.
Therefore the correct answer is an option (a)- $C{{H}_{3}}-C{{H}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}$.
Additional information:
There are 2 more isomers of straight-chain Bromopentane.
(i)- 1-Bromopentane: In which the 1st carbon atom has bromine substituent. The structure is given below:
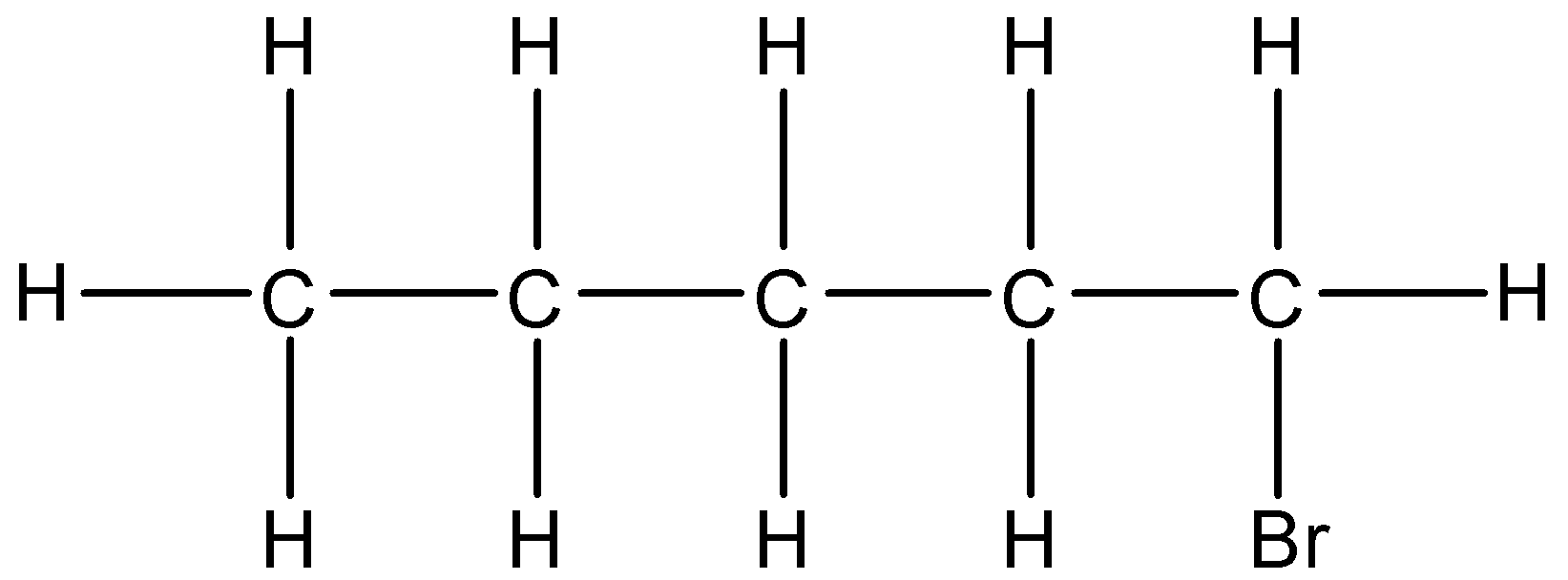
The condensed formula will be $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}(Br)$
(ii)- 2-Bromopentane: In which the 2nd carbon atom has bromine substituent. The structure is given below:
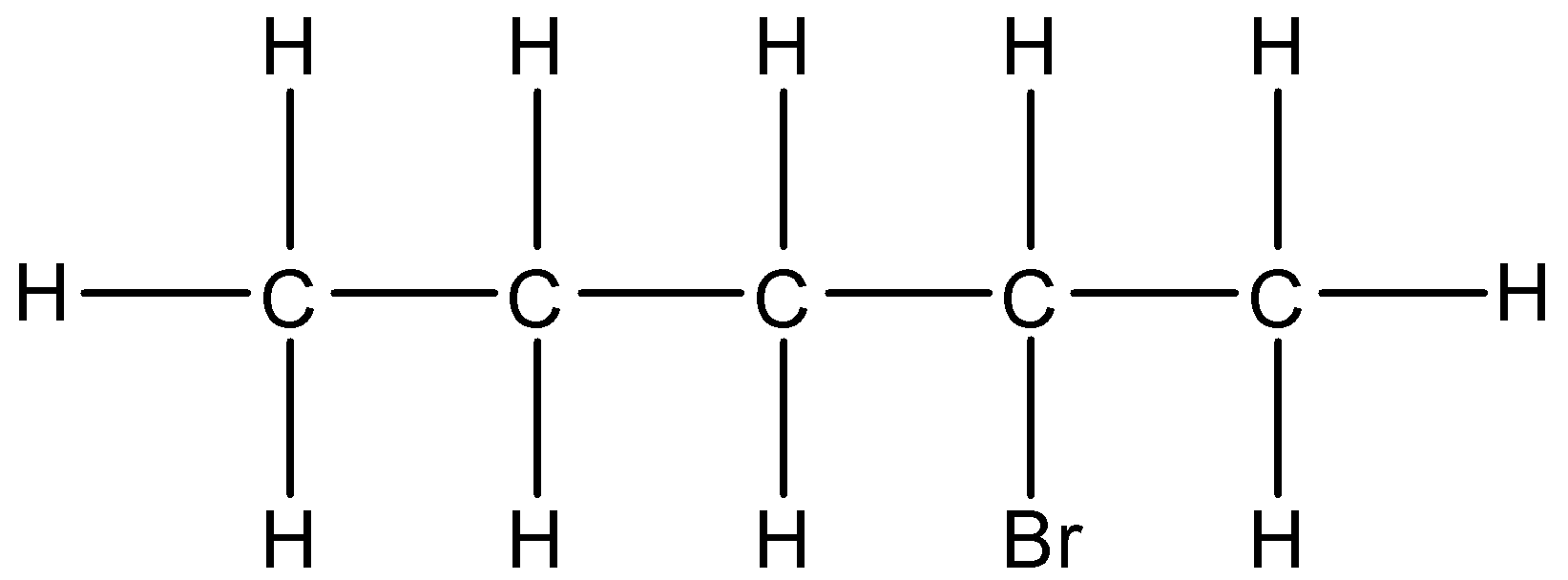
The condensed formula will be $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CH(Br)-C{{H}_{3}}$
Note: Other than straight-chain isomerism, Bromopentane also has chain isomers these are:
(i)- 1-Bromo-2-methyl butane: The structure is given below:
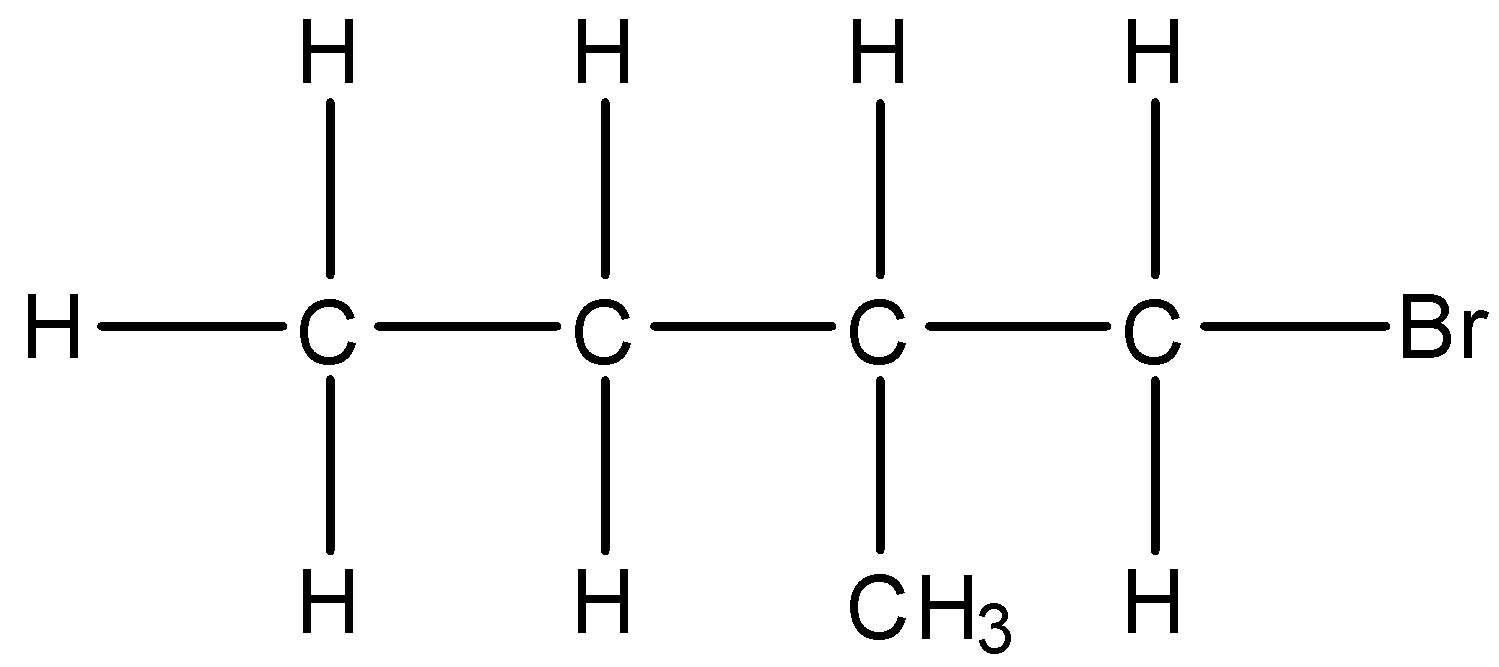
(ii)- 1-Bromo-3-methyl butane: The structure is given below:
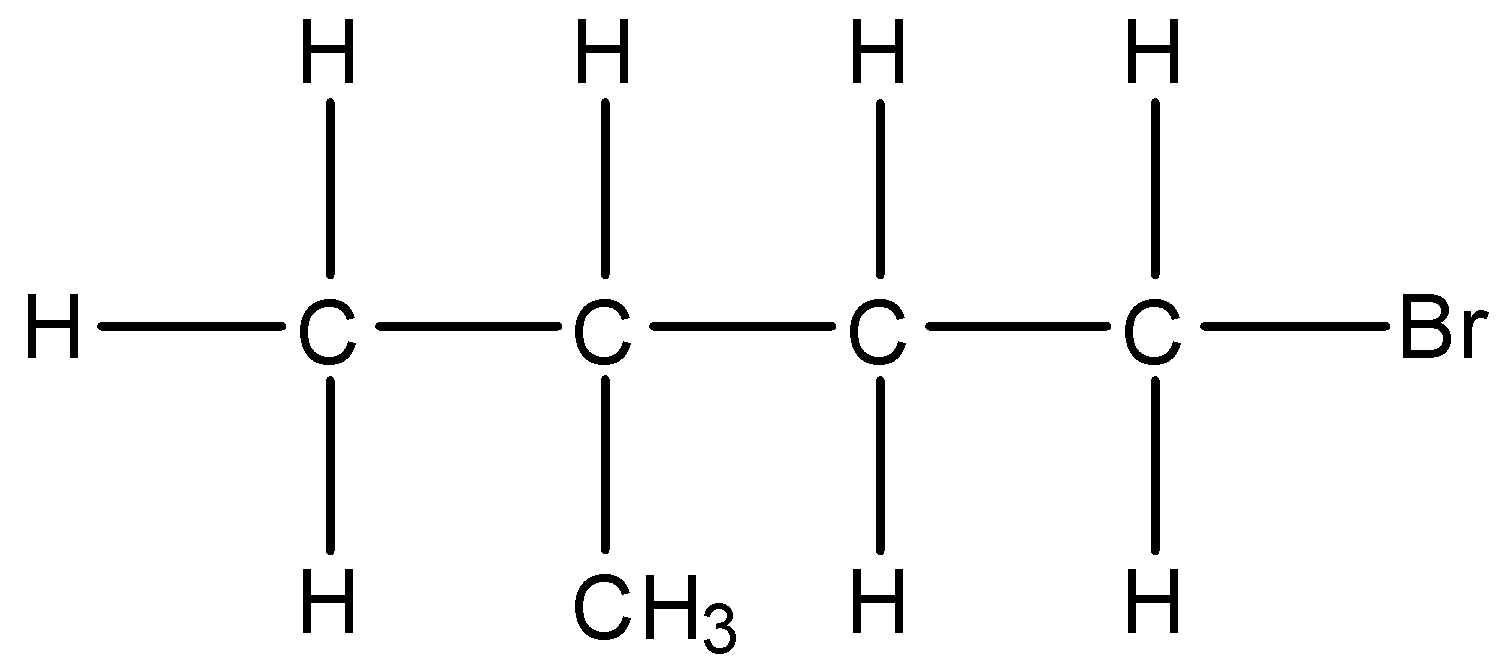
(iii)- 2-Bromo-2-methyl butane: The structure is given below:
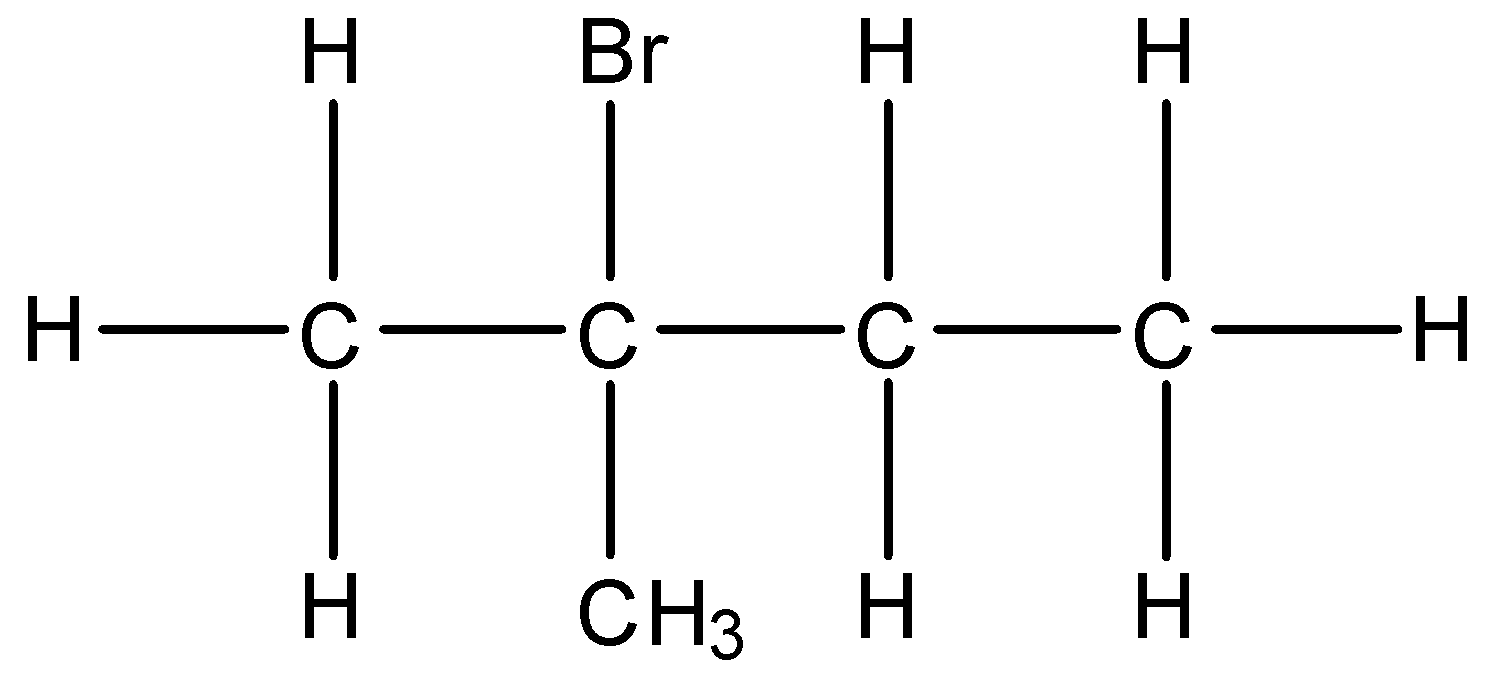
(iv)- 2-Bromo-3-methyl butane: The structure is given below:
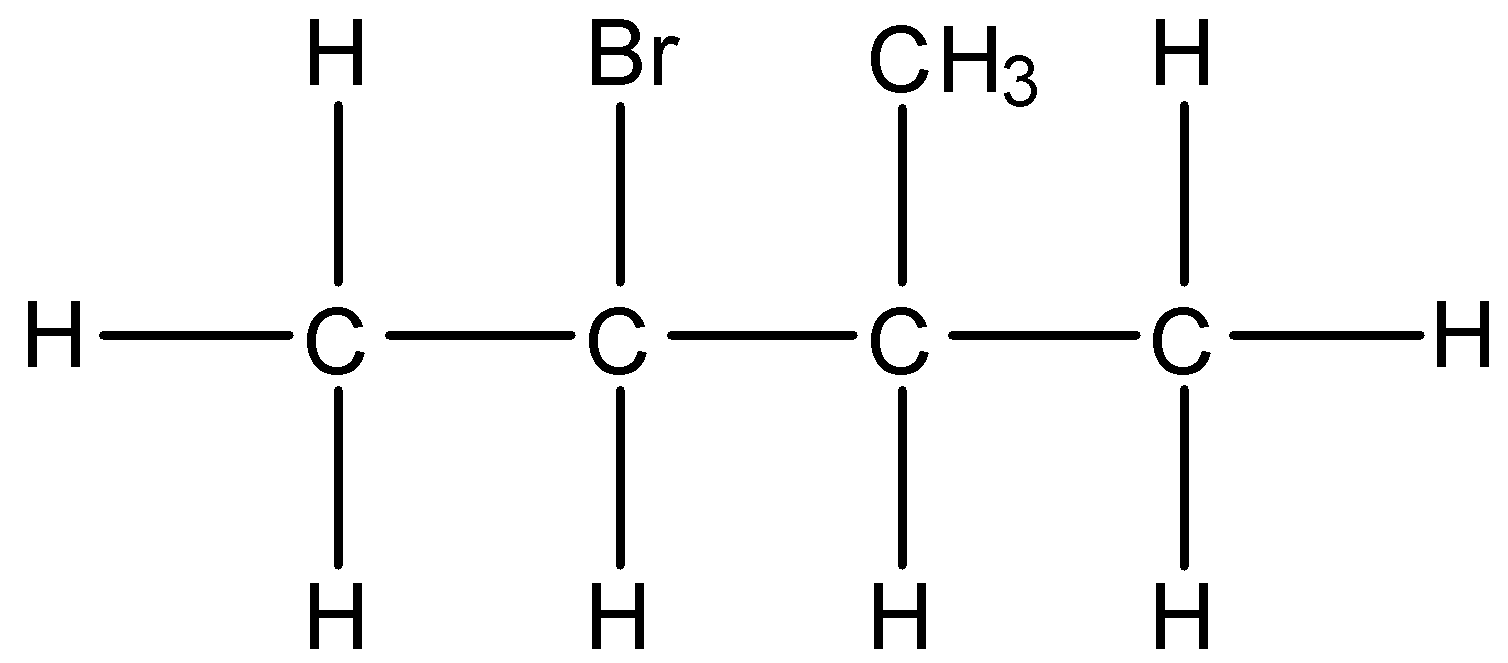
(v)- 1-Bromo-2,2-dimethylpropane: The structure is given below:
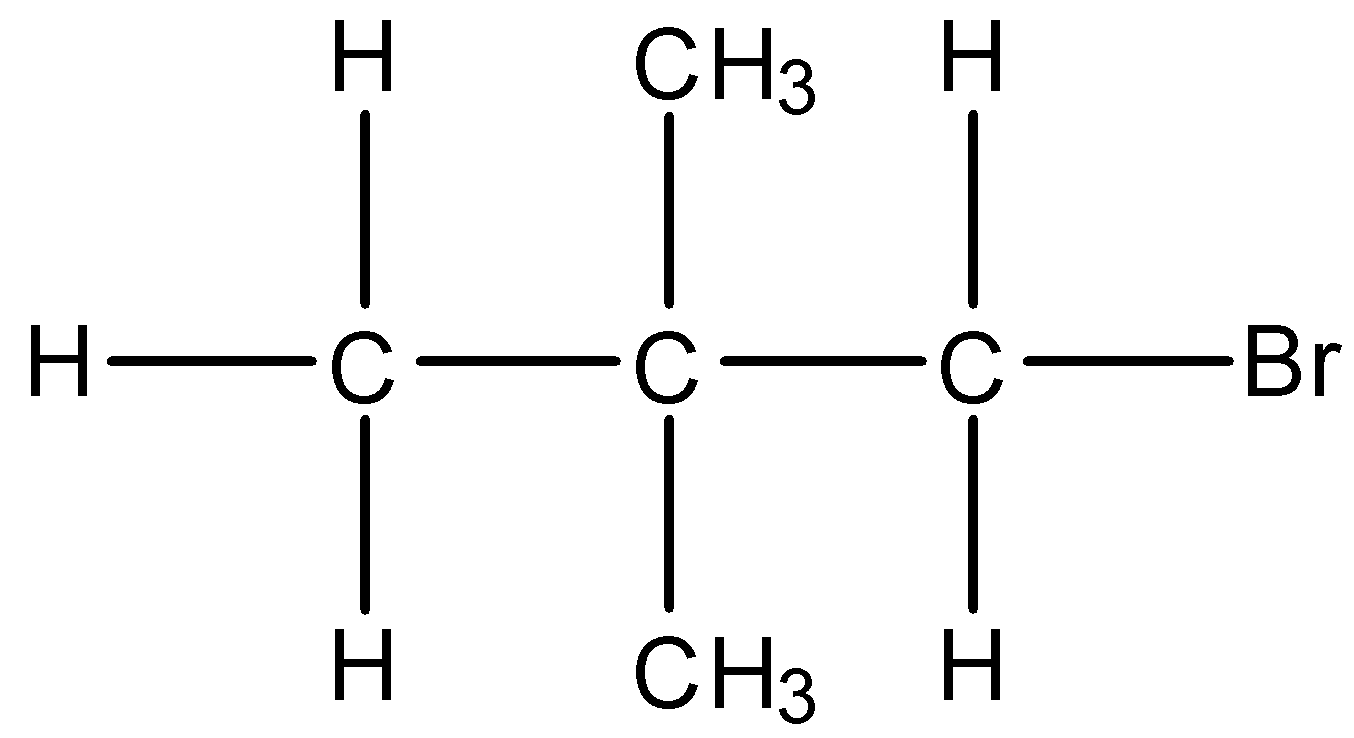
Complete step by step answer:
Pentane is the member of the homologous group called alkane. The general formula of alkanes is expressed as ${{C}_{n}}{{H}_{2n+2}}$.
The word penta means 5, so pentane must be having five carbon atoms present and pentane means, five carbon atoms in the alkane group.
When we put n as 5 in the general formula of alkane, we get:
${{C}_{5}}{{H}_{12}}$.
The structure of pentane is given below:

3-Bromopentane means the 3rd carbon atom must be having the bromine substituent. The bromine is attached to the carbon atom by substituting one hydrogen atom present at the 3rd carbon atom. The formula of 3-Bromopentane is\[{{C}_{5}}{{H}_{11}}Br\]. The expanded structure of 3-Bromopentane is given below:

So, in the condensed form we can write $C{{H}_{3}}-C{{H}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}$.
Therefore the correct answer is an option (a)- $C{{H}_{3}}-C{{H}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}$.
Additional information:
There are 2 more isomers of straight-chain Bromopentane.
(i)- 1-Bromopentane: In which the 1st carbon atom has bromine substituent. The structure is given below:

The condensed formula will be $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}(Br)$
(ii)- 2-Bromopentane: In which the 2nd carbon atom has bromine substituent. The structure is given below:

The condensed formula will be $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CH(Br)-C{{H}_{3}}$
Note: Other than straight-chain isomerism, Bromopentane also has chain isomers these are:
(i)- 1-Bromo-2-methyl butane: The structure is given below:

(ii)- 1-Bromo-3-methyl butane: The structure is given below:

(iii)- 2-Bromo-2-methyl butane: The structure is given below:

(iv)- 2-Bromo-3-methyl butane: The structure is given below:

(v)- 1-Bromo-2,2-dimethylpropane: The structure is given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




