
Draw the structural isomerism of $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ and name the type of isomerism.
Answer
592.5k+ views
Hint: In the given compound we have to determine the structural isomerism exhibited by it. Here, the ligand outside the coordination sphere can replace one of the similar ligands in the coordination sphere. Use this to find the solution.
Complete step by step answer:
To answer this, firstly let us discuss what isomerism is. Isomerism is the phenomenon where two or more chemical compounds have the same chemical formula but different chemical structures.
Isomerism can be further divided into two types- structural isomerism and stereoisomerism.
In the given question, we are asked about the structural isomers of $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ structural isomerism includes various subparts so let us go through them-
(a) Ionisation isomerism – This isomerism occurs due to interchange of ions between the inner and the outer coordination sphere. For example - $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Br \right]S{{O}_{4}}\text{ and }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}S{{O}_{4}} \right]Br$
(b) Hydration isomerism – This occurs due to different modes of distribution of water molecules between inner and outer coordination spheres. For example - $\left[ Cr{{\left( {{H}_{2}}O \right)}_{6}} \right]C{{l}_{3}}\text{ and }\left[ Cr{{\left( {{H}_{2}}O \right)}_{5}}Cl \right]C{{l}_{2}}\cdot {{H}_{2}}O$
(c) Coordination isomerism – When salts are produced by cationic and anionic compounds, metal centres between the complex entities can be interchanged to give coordination isomers. For example, ${{\left[ Pt{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2-}},{{\left[ PdC{{l}_{4}} \right]}^{2-}}$ can be inter-converted into ${{\left[ Pd{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2-}},{{\left[ PtC{{l}_{4}} \right]}^{2-}}$
(d) Ligand isomerism – This occurs due to isomeric nature of ligands, example-$N{{\left( C{{H}_{3}} \right)}_{3}}\text{ and C}{{\text{H}}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}$
(e) Linkage isomerism – This occurs due to the presence of ambidentate ligands having different donor sites. Example- $Co{{\left( SCN \right)}_{4}}^{2-}\text{ and }Co{{\left( NCS \right)}_{4}}^{2-}$
Now, let us see the complex given to us $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$.
We can see here that the $N{{O}_{2}}$ group is an ambidentate ligand i.e. it can either bond with the O-atom or the N-atom which will give us linkage isomerism.
Also, we can replace the $N{{O}_{2}}$ with one Cl atom present outside the coordination sphere and this will give us ionisation isomerism. Therefore we can write down the 3 possible isomers as- $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}},\text{ }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]N{{O}_{2}}Cl,\text{ }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}ONO \right]C{{l}_{2}}$
We can draw its structure as-
(i) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$, the only possible structure is-
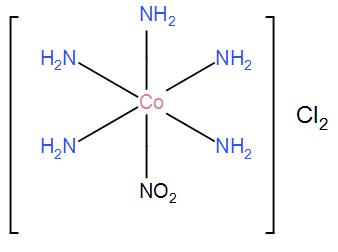
(ii) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}ONO \right]C{{l}_{2}}$, the only possible structure is-
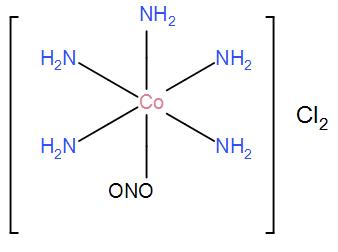
(iii) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]N{{O}_{2}}Cl$, the only possible structure is-
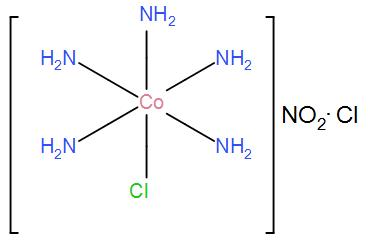
Therefore, the required answer is the above structures and the name of the possible isomers is – linkage and ionization.
Note: We have mentioned above that isomerism is of two types: stereoisomers and structural.
Stereo isomerism is of two types-
(a) Geometrical isomerism – These are the compounds with the same number and types of atoms but have different geometries. For example- fumaric acid and maleic acid have the same number and type of atoms but they are not the same.
(b) Optical isomerism – These are two compounds with the same number of atoms with the same bond connectivity but their spatial arrangement is different.
Complete step by step answer:
To answer this, firstly let us discuss what isomerism is. Isomerism is the phenomenon where two or more chemical compounds have the same chemical formula but different chemical structures.
Isomerism can be further divided into two types- structural isomerism and stereoisomerism.
In the given question, we are asked about the structural isomers of $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ structural isomerism includes various subparts so let us go through them-
(a) Ionisation isomerism – This isomerism occurs due to interchange of ions between the inner and the outer coordination sphere. For example - $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Br \right]S{{O}_{4}}\text{ and }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}S{{O}_{4}} \right]Br$
(b) Hydration isomerism – This occurs due to different modes of distribution of water molecules between inner and outer coordination spheres. For example - $\left[ Cr{{\left( {{H}_{2}}O \right)}_{6}} \right]C{{l}_{3}}\text{ and }\left[ Cr{{\left( {{H}_{2}}O \right)}_{5}}Cl \right]C{{l}_{2}}\cdot {{H}_{2}}O$
(c) Coordination isomerism – When salts are produced by cationic and anionic compounds, metal centres between the complex entities can be interchanged to give coordination isomers. For example, ${{\left[ Pt{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2-}},{{\left[ PdC{{l}_{4}} \right]}^{2-}}$ can be inter-converted into ${{\left[ Pd{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2-}},{{\left[ PtC{{l}_{4}} \right]}^{2-}}$
(d) Ligand isomerism – This occurs due to isomeric nature of ligands, example-$N{{\left( C{{H}_{3}} \right)}_{3}}\text{ and C}{{\text{H}}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}$
(e) Linkage isomerism – This occurs due to the presence of ambidentate ligands having different donor sites. Example- $Co{{\left( SCN \right)}_{4}}^{2-}\text{ and }Co{{\left( NCS \right)}_{4}}^{2-}$
Now, let us see the complex given to us $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$.
We can see here that the $N{{O}_{2}}$ group is an ambidentate ligand i.e. it can either bond with the O-atom or the N-atom which will give us linkage isomerism.
Also, we can replace the $N{{O}_{2}}$ with one Cl atom present outside the coordination sphere and this will give us ionisation isomerism. Therefore we can write down the 3 possible isomers as- $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}},\text{ }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]N{{O}_{2}}Cl,\text{ }\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}ONO \right]C{{l}_{2}}$
We can draw its structure as-
(i) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$, the only possible structure is-

(ii) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}ONO \right]C{{l}_{2}}$, the only possible structure is-

(iii) For $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}Cl \right]N{{O}_{2}}Cl$, the only possible structure is-

Therefore, the required answer is the above structures and the name of the possible isomers is – linkage and ionization.
Note: We have mentioned above that isomerism is of two types: stereoisomers and structural.
Stereo isomerism is of two types-
(a) Geometrical isomerism – These are the compounds with the same number and types of atoms but have different geometries. For example- fumaric acid and maleic acid have the same number and type of atoms but they are not the same.
(b) Optical isomerism – These are two compounds with the same number of atoms with the same bond connectivity but their spatial arrangement is different.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

Write the formula to find the shortest distance between class 12 maths CBSE




