
Draw the shape of the $ CI{{F}_{}}^{+} $ ions, including any lone pairs, and name the shape made by the atoms? Predict the bond angle in the ion?
Answer
491.1k+ views
Hint: $ CI{{F}_{}}^{+} $ is referred to as difluorochloranium. It is formed when $ CI{{F}_{3}} $ loses one fluorine atom. It gains a positive charge from the removal of one cation from the $ CI{{F}_{3}} $ structure. The shape of $ CI{{F}_{}}^{+} $ will differ from that of $ CI{{F}_{3}} $ the compound. Also, the bond angle will reduce in the unipositive molecule.
Complete answer:
$ CI{{F}_{}}^{+} $ has two fluorine atoms and a central chlorine atom. Firstly, we will draw the Lewis structure $ CI{{F}_{}}^{+} $ . The positive charge will be positioned on Chlorine. Chlorine will be in the centre with the two fluorine atoms on either side.
Let’s take into account all the electrons in the structure. Chlorine has 7 atoms in the valence shell and so do fluorine atoms. So, the total electrons from one chlorine and two fluorine result in 21 electrons. But as it has a positive charge so an electron will be deducted from the total valence electrons.
$ \therefore \text{ Total No}\text{. of }{{\text{e}}^{-}}\text{ = No}\text{. of }{{\text{e}}^{-}}\text{ on Cl + 2 }\times \text{ No}\text{. of }{{\text{e}}^{-}}\text{ on }{{\text{F}}^{-}} $
$ =\text{ 7 + 2 }\times \text{ 7 - 1} $
$ =\text{ 20 }{{\text{e}}^{-}} $
Total electrons are 20, so the lone pairs will be 10 in $ CI{{F}_{}}^{+} $ . Two electrons are shared between each atom to form a bond. Thus, each atom will complete its octet of electrons.
The Lewis structure of $ CI{{F}_{}}^{+} $ is as follows:
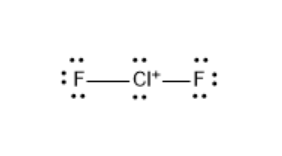
$ CI{{F}_{}}^{+} $ has three lone pairs each on fluoride atoms and two lone pairs on chlorine atoms. Also, 2 bonding pairs are used to make two F-Cl bonds.
The shape of the ion is determined by the VSEPR theory. The central atom possesses 2 lone pairs and 2 bonding pairs i.e. 4 electrons in each domain. Thus according to the VSEPR theory, the electron geometry is tetrahedral.
However, the molecular geometry only includes the bonding pairs so the molecular shape is ‘bent’ with a theoretical bond angle of $109.5^\circ$.
Note:
The actual bond angle comes out to be 104.5o. But due to the repulsion caused by the lone pair of electrons on the central atom the F-Cl-F bond angle changes to 109.5o. The electron cloud is placed at an angle above the chlorine atom to cause minimum repulsion.
Complete answer:
$ CI{{F}_{}}^{+} $ has two fluorine atoms and a central chlorine atom. Firstly, we will draw the Lewis structure $ CI{{F}_{}}^{+} $ . The positive charge will be positioned on Chlorine. Chlorine will be in the centre with the two fluorine atoms on either side.
Let’s take into account all the electrons in the structure. Chlorine has 7 atoms in the valence shell and so do fluorine atoms. So, the total electrons from one chlorine and two fluorine result in 21 electrons. But as it has a positive charge so an electron will be deducted from the total valence electrons.
$ \therefore \text{ Total No}\text{. of }{{\text{e}}^{-}}\text{ = No}\text{. of }{{\text{e}}^{-}}\text{ on Cl + 2 }\times \text{ No}\text{. of }{{\text{e}}^{-}}\text{ on }{{\text{F}}^{-}} $
$ =\text{ 7 + 2 }\times \text{ 7 - 1} $
$ =\text{ 20 }{{\text{e}}^{-}} $
Total electrons are 20, so the lone pairs will be 10 in $ CI{{F}_{}}^{+} $ . Two electrons are shared between each atom to form a bond. Thus, each atom will complete its octet of electrons.
The Lewis structure of $ CI{{F}_{}}^{+} $ is as follows:

$ CI{{F}_{}}^{+} $ has three lone pairs each on fluoride atoms and two lone pairs on chlorine atoms. Also, 2 bonding pairs are used to make two F-Cl bonds.
The shape of the ion is determined by the VSEPR theory. The central atom possesses 2 lone pairs and 2 bonding pairs i.e. 4 electrons in each domain. Thus according to the VSEPR theory, the electron geometry is tetrahedral.
However, the molecular geometry only includes the bonding pairs so the molecular shape is ‘bent’ with a theoretical bond angle of $109.5^\circ$.
Note:
The actual bond angle comes out to be 104.5o. But due to the repulsion caused by the lone pair of electrons on the central atom the F-Cl-F bond angle changes to 109.5o. The electron cloud is placed at an angle above the chlorine atom to cause minimum repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




