
Draw the pyranose structure of glucose.
Answer
542.1k+ views
Hint: The pyranose structure of glucose is similar to the organic compound pyran, which is a six membered ring with one oxygen and five carbon atoms in the ring.
Complete answer:
In order to answer the question, we need to learn about the structure and properties of glucose.
Cyclic structure of glucose: The limitations shown by the open chain structure of glucose can be explained by its cyclic structure. It was proposed that glucose can form a six-membered ring in which $-OH$ at C-5 can add to the $-CHO$ group and can form a cyclic hemiacetal structure. This explains the absence of $-CHO$ group and also the existence of glucose in $\alpha ,\beta $ forms.
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C-1, called anomeric carbon (the aldehyde carbon before cyclisation) and the corresponding $\alpha $ and $\beta $ forms are called anomers. It should be noted that a and B-forms of glucose are not mirror images of each other, hence are not enantiomers. The six membered cyclic structure of glucose is called pyranose structure ($\alpha $ or $\beta $), in analogy with pyran.
Pyran is a six membered ring with one oxygen and five carbon atoms in the ring. The cyclic structure of glucose can be more accurately shown by Haworth structure as given below:
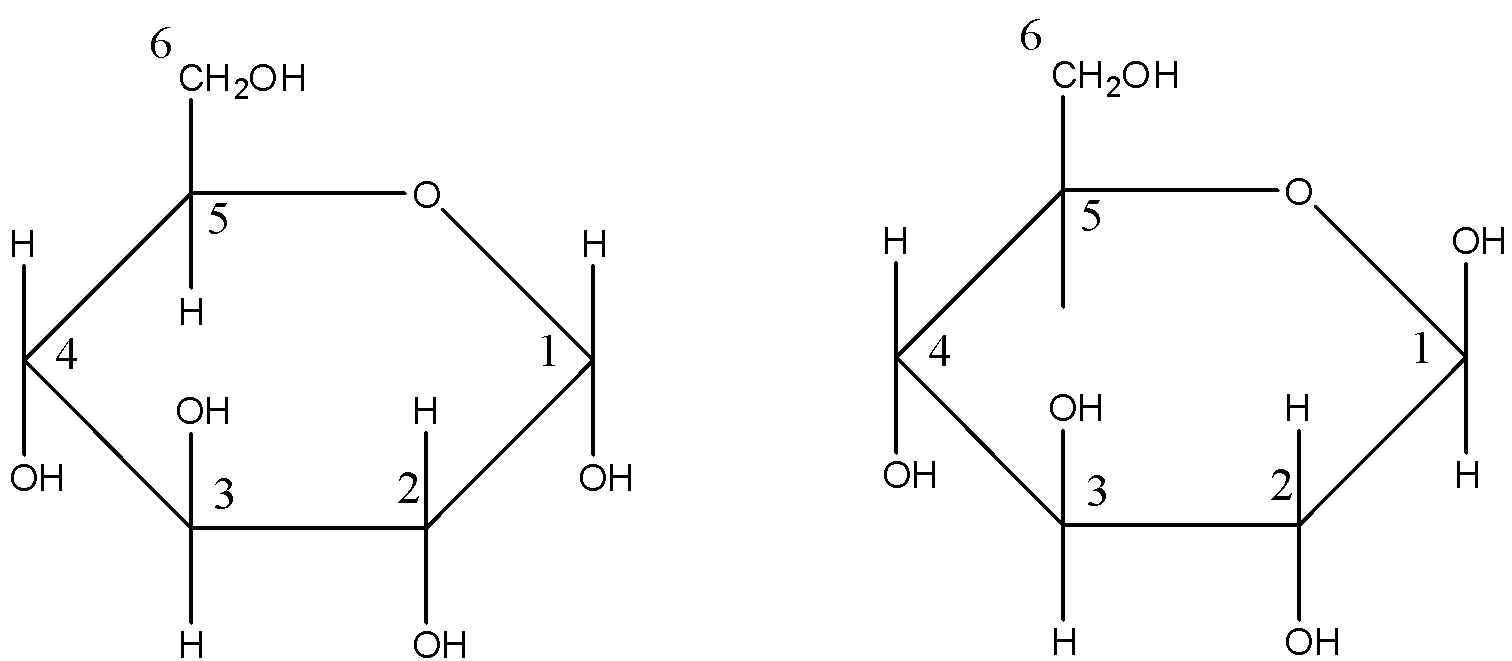
The left diagram shows the structure of $\alpha $-D-( + ) Glucopyranose and the right diagram shows the structure of $\beta $-D-( + ) Glucopyranose.
Note:
It is to be noted that the cyclic structure of glucose was so given because the existence of glucose in $\alpha ,\beta $ forms could not be explained by it’s open chain structure.
Complete answer:
In order to answer the question, we need to learn about the structure and properties of glucose.
Cyclic structure of glucose: The limitations shown by the open chain structure of glucose can be explained by its cyclic structure. It was proposed that glucose can form a six-membered ring in which $-OH$ at C-5 can add to the $-CHO$ group and can form a cyclic hemiacetal structure. This explains the absence of $-CHO$ group and also the existence of glucose in $\alpha ,\beta $ forms.
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C-1, called anomeric carbon (the aldehyde carbon before cyclisation) and the corresponding $\alpha $ and $\beta $ forms are called anomers. It should be noted that a and B-forms of glucose are not mirror images of each other, hence are not enantiomers. The six membered cyclic structure of glucose is called pyranose structure ($\alpha $ or $\beta $), in analogy with pyran.
Pyran is a six membered ring with one oxygen and five carbon atoms in the ring. The cyclic structure of glucose can be more accurately shown by Haworth structure as given below:

The left diagram shows the structure of $\alpha $-D-( + ) Glucopyranose and the right diagram shows the structure of $\beta $-D-( + ) Glucopyranose.
Note:
It is to be noted that the cyclic structure of glucose was so given because the existence of glucose in $\alpha ,\beta $ forms could not be explained by it’s open chain structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




