
How do you draw the product of the hydrogenation of ethyne?
Answer
533.4k+ views
Hint: We know that the hydrogenation reaction involves the direct reaction of a substrate containing $ \pi - $ bonds or $ \pi - $ bond like nature with $ {{H}_{2}} $ molecule using a metal like Palladium $ \left( Pd \right) $ , Platinum $ \left( Pt \right) $ , Rhodium $ \left( Rh \right) $ or Nickel $ \left( Ni \right) $ as a base of support for the reaction giving rise to products which may be partially hydrogenated or completely hydrogenated.
Complete step by step answer:
When we treat ethyne which is a chemical species represented by $ HC\equiv CH $ and contains $ 2\pi -bonds $ with a hydrogenating reagent, the product we would obtain is a cis-hydrogenation product always. The one difference however that we may obtain in the product is the number of $ \pi -bonds $ left in them, depending on the reagent used we may obtain an ethene $ \left( C{{H}_{2}}=C{{H}_{2}} \right) $ or an ethane $ \left( C{{H}_{3}}-C{{H}_{3}} \right) $ as the final product. An illustration to show this is provided below:-
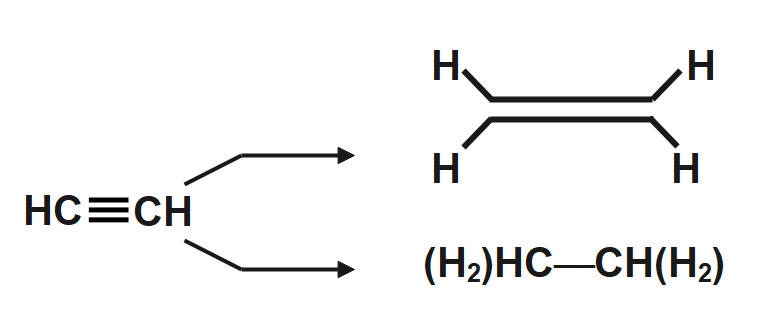
The Hydrogens shown in orange are the hydrogens that are obtained from $ {{H}_{2}} $ as you can clearly see the hydrogenation occurs in a cis-manner as seen clear in the product $ \left[ A \right] $ .
Depending on what reagent has been used the partial hydrogenation product $ \left[ A \right] $ or the complete hydrogenation product $ \left[ B \right] $ is obtained. So we must be clearly wary about the reaction conditions and draw the products required accordingly. To help understand the process of hydrogenation in an even more clear manner let us look at the illustration below:
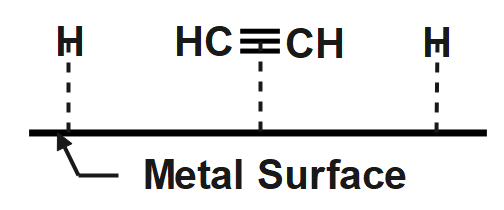
Note: Note that generally for partial hydrogenation a catalyst is poisoned using some chemical species which reduces its reactivity so take note on every species present also when forming ethane you don’t need to show cis-attack as in case of ethane due to presence of only a single bond it can easily rotate and thus no purely cis or trans species can be obtained for ethane.
Complete step by step answer:
When we treat ethyne which is a chemical species represented by $ HC\equiv CH $ and contains $ 2\pi -bonds $ with a hydrogenating reagent, the product we would obtain is a cis-hydrogenation product always. The one difference however that we may obtain in the product is the number of $ \pi -bonds $ left in them, depending on the reagent used we may obtain an ethene $ \left( C{{H}_{2}}=C{{H}_{2}} \right) $ or an ethane $ \left( C{{H}_{3}}-C{{H}_{3}} \right) $ as the final product. An illustration to show this is provided below:-

The Hydrogens shown in orange are the hydrogens that are obtained from $ {{H}_{2}} $ as you can clearly see the hydrogenation occurs in a cis-manner as seen clear in the product $ \left[ A \right] $ .
Depending on what reagent has been used the partial hydrogenation product $ \left[ A \right] $ or the complete hydrogenation product $ \left[ B \right] $ is obtained. So we must be clearly wary about the reaction conditions and draw the products required accordingly. To help understand the process of hydrogenation in an even more clear manner let us look at the illustration below:

Note: Note that generally for partial hydrogenation a catalyst is poisoned using some chemical species which reduces its reactivity so take note on every species present also when forming ethane you don’t need to show cis-attack as in case of ethane due to presence of only a single bond it can easily rotate and thus no purely cis or trans species can be obtained for ethane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




