
Draw the molecular structure of\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\].
Answer
588k+ views
Hint: The dinitrogen pentoxide\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]is a challenging structure. The Lewis dot structure contains the 40 valence electrons and thus a total of 20 electron-pair to accommodate the structure. This electron-pair can be sigma bonds, pi bonds, or lone pairs. Each oxygen completes its content by accommodating two pairs of two oxygen and nitrogen and completing its octet by forming a pi bond with the adjacent oxygen atom.
Complete step by step answer:
This \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\] is a bit challenging molecule to draw.
First, we will sketch the basic structure \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]as shown below. Now we are going to find out how the σ bonds, π bonds, and lone pairs are arranged in the molecule.
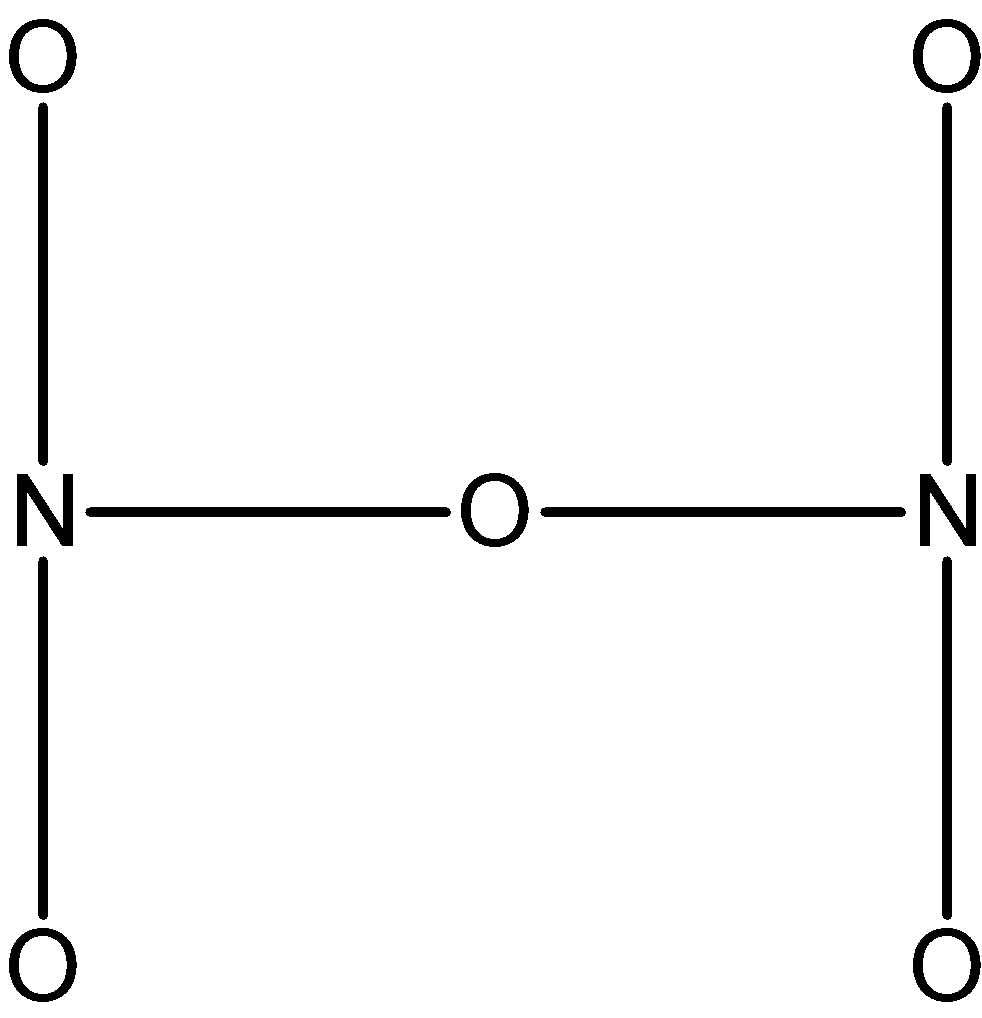
The structure is arranged such that the 5 oxygen and 2 nitrogen are forming a skeleton\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\].
In\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\],
The total number of the valence electron in the nitrogen atom $=\text{ 5}$
The Number of a nitrogen atom in the \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]molecules $=\text{ 2}$
Therefore, the total number of valence electron from two nitrogen atoms are, $\text{ 5 }\times \text{ 2 =10 }{{\text{e}}^{-}}$
Now, each nitrogen is bonded to the oxygen.
The number of the valence electron in the oxygen atom $=\text{ 6}$
The number of oxygen in the \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]molecule atom $=\text{ 5}$
Therefore, the total number of an electron from the two oxygen atoms are, $\text{ 6 }\times \text{ 5 = 30 }{{\text{e}}^{-}}$
Thus, the total number of electrons in the valence shell of each atom is,
$\begin{align}
& \text{ = 10 + 30} \\
& \text{ = 40 }{{\text{e}}^{-}} \\
\end{align}$
Now, we can calculate the total number of pairs of the electron. To do so, divide the total valence electron by the 2. we get,
$\text{ number of electron pair = }\frac{40\text{ }{{e}^{-}}}{2}\text{ = 20 pairs}$
There are 20 electron pairs in\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]. Thus 20 electron pairs may be σ bonds, π bonds, or lone pairs.
Now, we will start to draw the Lewis dot structure for\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]. In the above skeleton we have already marked 5 σ bonds.so we are left with 15 electron pairs. Let's mark the rest of the electron pair as the lone pairs of oxygen. The lone pairs are updated as follows,
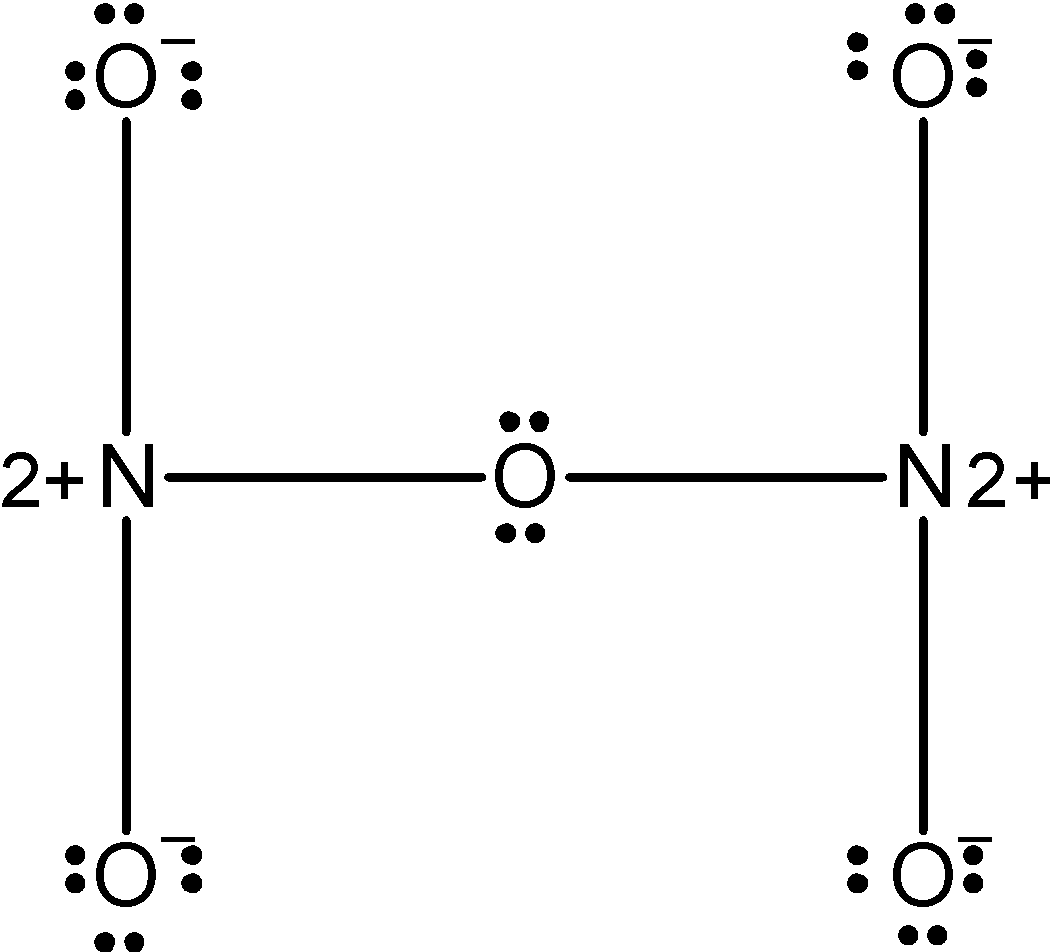
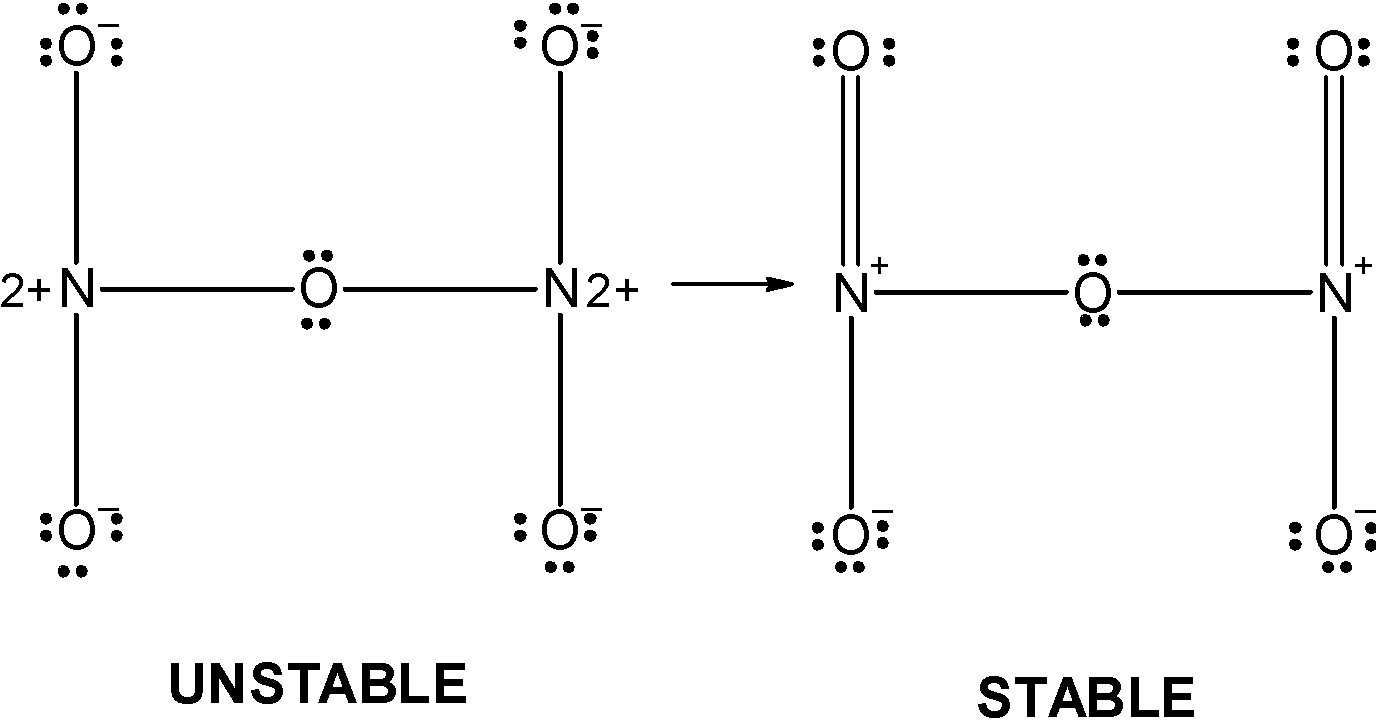
The 4 oxygen has the 8 electrons in their valence shell. They possess the $\text{ -1}$ charge. The one oxygen which is in between the two nitrogen atoms has completed its valency and thus it has zero charges on it. The structure depicts that the octet of the nitrogen is not complete since both of them acquire the $\text{ }+2$ charge. Therefore the above structure is wrong and unstable therefore we can reduce the charge on the atoms by converting the lone pairs on the oxygen by the pi bonds. The modified structure is as follows,
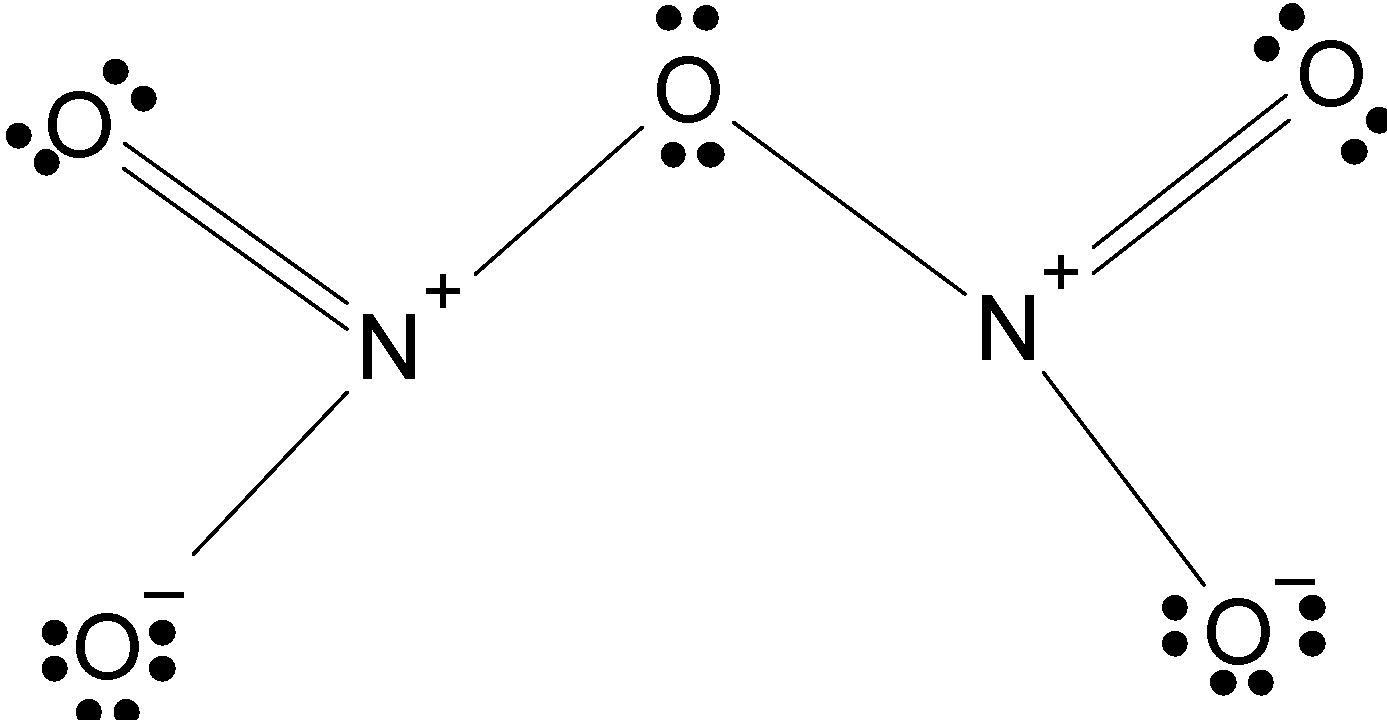
Therefore, the correct stable molecular structure of \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\] is
Note: The structure contains the 40 valence electron therefore it is challenging to draw the structure. The key is to place oxygen atoms between the nitrogen atoms and attach the four atoms to the nitrogen atom. This is an exception to the “least electronegative atom at the centre rule”. Thus makes its structure tricky.
Complete step by step answer:
This \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\] is a bit challenging molecule to draw.
First, we will sketch the basic structure \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]as shown below. Now we are going to find out how the σ bonds, π bonds, and lone pairs are arranged in the molecule.

The structure is arranged such that the 5 oxygen and 2 nitrogen are forming a skeleton\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\].
In\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\],
The total number of the valence electron in the nitrogen atom $=\text{ 5}$
The Number of a nitrogen atom in the \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]molecules $=\text{ 2}$
Therefore, the total number of valence electron from two nitrogen atoms are, $\text{ 5 }\times \text{ 2 =10 }{{\text{e}}^{-}}$
Now, each nitrogen is bonded to the oxygen.
The number of the valence electron in the oxygen atom $=\text{ 6}$
The number of oxygen in the \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]molecule atom $=\text{ 5}$
Therefore, the total number of an electron from the two oxygen atoms are, $\text{ 6 }\times \text{ 5 = 30 }{{\text{e}}^{-}}$
Thus, the total number of electrons in the valence shell of each atom is,
$\begin{align}
& \text{ = 10 + 30} \\
& \text{ = 40 }{{\text{e}}^{-}} \\
\end{align}$
Now, we can calculate the total number of pairs of the electron. To do so, divide the total valence electron by the 2. we get,
$\text{ number of electron pair = }\frac{40\text{ }{{e}^{-}}}{2}\text{ = 20 pairs}$
There are 20 electron pairs in\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]. Thus 20 electron pairs may be σ bonds, π bonds, or lone pairs.
Now, we will start to draw the Lewis dot structure for\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]. In the above skeleton we have already marked 5 σ bonds.so we are left with 15 electron pairs. Let's mark the rest of the electron pair as the lone pairs of oxygen. The lone pairs are updated as follows,

The 4 oxygen has the 8 electrons in their valence shell. They possess the $\text{ -1}$ charge. The one oxygen which is in between the two nitrogen atoms has completed its valency and thus it has zero charges on it. The structure depicts that the octet of the nitrogen is not complete since both of them acquire the $\text{ }+2$ charge. Therefore the above structure is wrong and unstable therefore we can reduce the charge on the atoms by converting the lone pairs on the oxygen by the pi bonds. The modified structure is as follows,

Therefore, the correct stable molecular structure of \[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\] is

Note: The structure contains the 40 valence electron therefore it is challenging to draw the structure. The key is to place oxygen atoms between the nitrogen atoms and attach the four atoms to the nitrogen atom. This is an exception to the “least electronegative atom at the centre rule”. Thus makes its structure tricky.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




