
Draw the molecular orbital energy level diagram of ${N_2}$ molecules.
Answer
582.6k+ views
Hint: The number of electrons present in the bonding orbitals is represented by \[{N_b}\] and the number of electrons present in antibonding orbitals by Na
Complete step by step answer:
Energy level diagrams are a means of analysing the energies electrons can accept and release as they transition from one accepted orbital to another
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, \[2{p_x}\] , \[2{p_y}\] , and \[2{p_z}\] ). In the Lewis structure there is a triple bond between the nitrogen atoms and a nonbonding pair of electrons on each. This is consistent with the physical properties of ${N_2}$ .
$\sigma *2p$

$\sigma 2{s^*}$
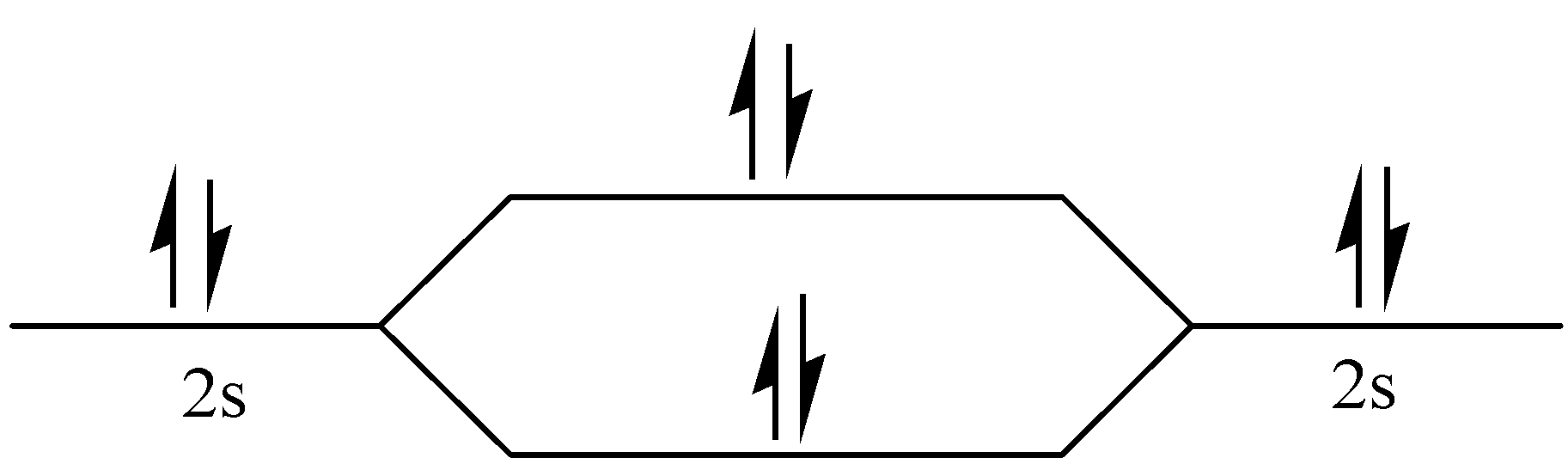
$\sigma 2s$
$\sigma 1{s^*}$
σ1s
Figure – The molecular orbital energy level diagram of ${N_2}$ molecules
Let me explain the molecular orbital diagram of ${N_2}$ using its diagram.
One atom of nitrogen has 7 electrons so a ${N_2}$ molecule will have 14 electrons, so first 2 electrons go in 1s sigma bond, the next 2 in 1s sigma anti bond orbital, next 2 in 2s sigma bond orbital, next 2 in 2s sigma anti bond orbital, next 2 in 2pz sigma bond (assuming that z axis is the internuclear axis) orbital and next 4 in 2p pi x and 2 2p pi y orbitals
Number of bonding electrons:10 Number of anti-bonds: 4
bond order: \[\dfrac{{\left( {10-4} \right)}}{2} = 3\]
This shows that ${N_2}$ has a triple covalent bond. Since, all the electrons in nitrogen are paired, it is a diamagnetic molecule.
Note: Formation of molecular orbitals can be determined by LCAO (linear combination of atomic orbitals) method.
Complete step by step answer:
Energy level diagrams are a means of analysing the energies electrons can accept and release as they transition from one accepted orbital to another
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, \[2{p_x}\] , \[2{p_y}\] , and \[2{p_z}\] ). In the Lewis structure there is a triple bond between the nitrogen atoms and a nonbonding pair of electrons on each. This is consistent with the physical properties of ${N_2}$ .
$\sigma *2p$

$\sigma 2{s^*}$

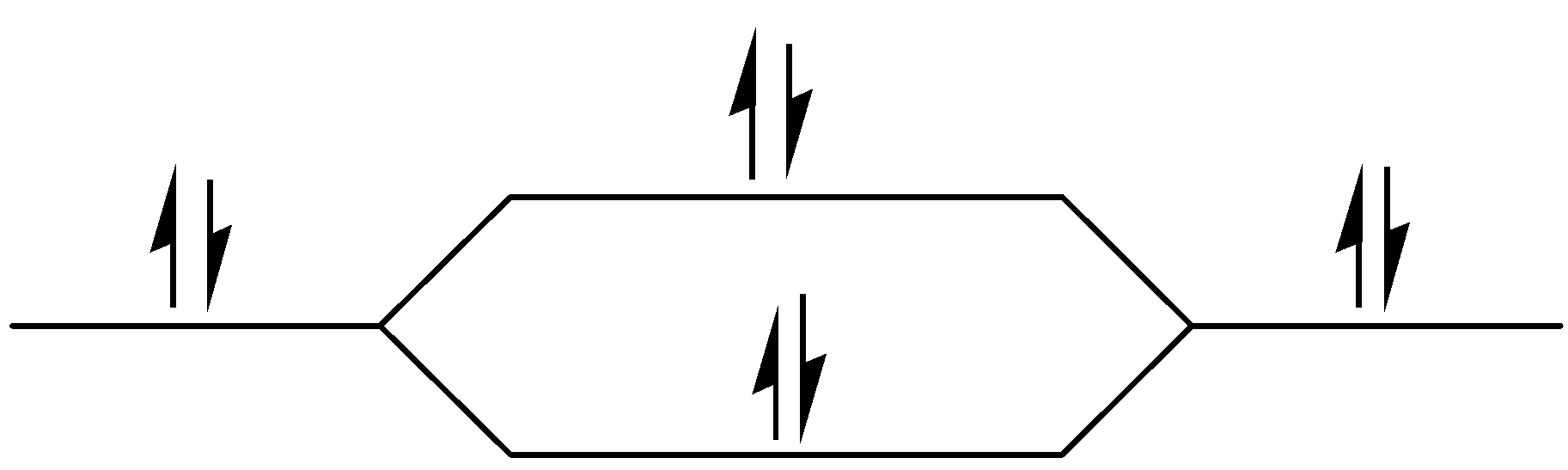
$\sigma 2s$
$\sigma 1{s^*}$

σ1s
Figure – The molecular orbital energy level diagram of ${N_2}$ molecules
Let me explain the molecular orbital diagram of ${N_2}$ using its diagram.
One atom of nitrogen has 7 electrons so a ${N_2}$ molecule will have 14 electrons, so first 2 electrons go in 1s sigma bond, the next 2 in 1s sigma anti bond orbital, next 2 in 2s sigma bond orbital, next 2 in 2s sigma anti bond orbital, next 2 in 2pz sigma bond (assuming that z axis is the internuclear axis) orbital and next 4 in 2p pi x and 2 2p pi y orbitals
Number of bonding electrons:10 Number of anti-bonds: 4
bond order: \[\dfrac{{\left( {10-4} \right)}}{2} = 3\]
This shows that ${N_2}$ has a triple covalent bond. Since, all the electrons in nitrogen are paired, it is a diamagnetic molecule.
Note: Formation of molecular orbitals can be determined by LCAO (linear combination of atomic orbitals) method.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




