
How do you draw the Lewis structure of phosphorus pentachloride?
Answer
564.9k+ views
Hint: The lewis dot structure tells the number of valence electrons around the symbol of the element. The number of valence electrons in the phosphorus is 5 and the number of valence electrons in chlorine is 7, and one bond is formed by two electrons in which one-one electrons are donated by each atom.
Complete answer:
The lewis dot structure tells the number of valence electrons around the symbol of the element. According to the number of electrons in the atoms are arranged in the shells and the last shell of the atom is the valence shell and the electrons in that shell are valence electrons and these valence electrons are responsible for the bond formation.
The number of valence electrons in the phosphorus is 5 and the number of valence electrons of chlorine is 7, and one bond is formed by two electrons in which one-one electrons are donated by each atom. The bonds must be formed in such a way that each atom can complete its octet.
There must be five dots around the phosphorus atom and there must be seven electrons around the chlorine atom.
In phosphorus pentachloride, there is one phosphorus atom and there are five chlorine atoms. So, the total electrons in the Lewis dot structure will be:
$5+7+7+7+7+7=40$
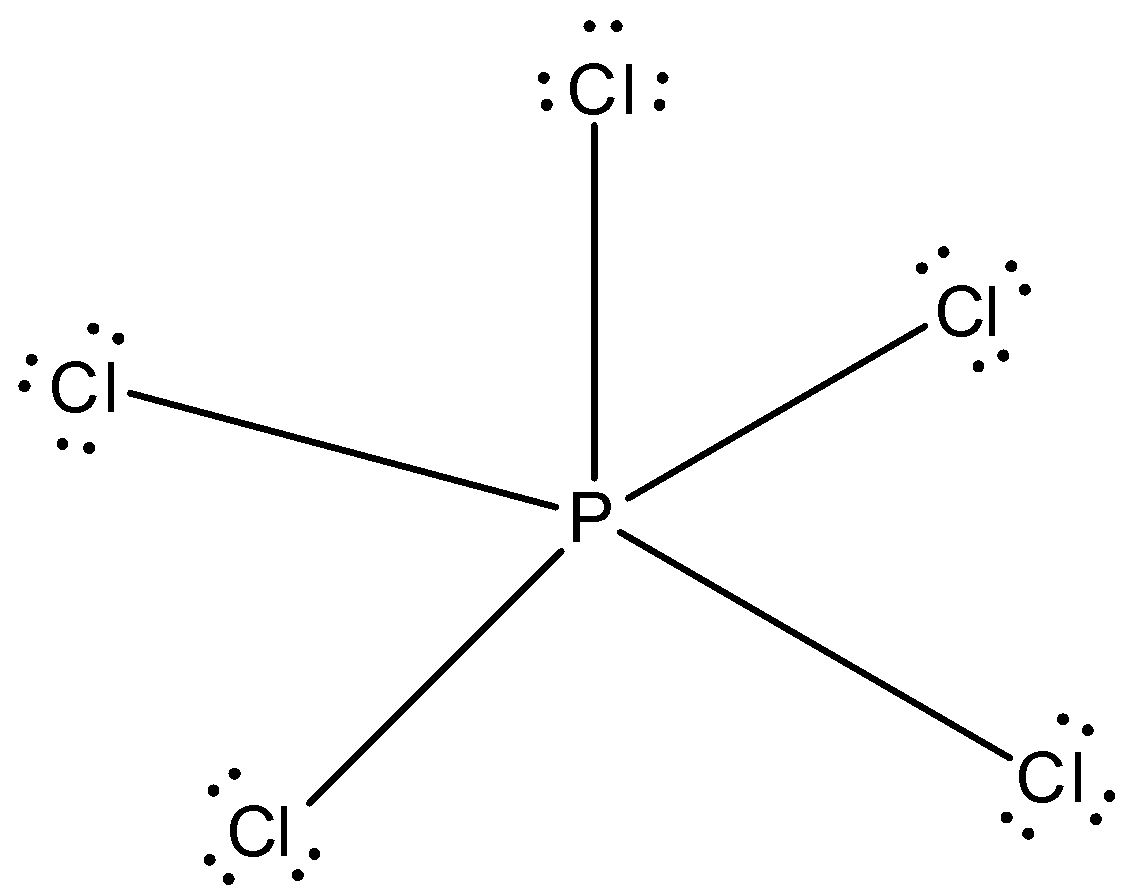
All the electrons of phosphorus are bonded to chlorine atom and the structure is given below:
Note:
If the overall charge on the molecule is negative then the electron is added to the molecule and if the overall charge on the molecule is positive then the electrons are deducted from the molecule. As the charge increases the number of electrons gained or lost also increases.
Complete answer:
The lewis dot structure tells the number of valence electrons around the symbol of the element. According to the number of electrons in the atoms are arranged in the shells and the last shell of the atom is the valence shell and the electrons in that shell are valence electrons and these valence electrons are responsible for the bond formation.
The number of valence electrons in the phosphorus is 5 and the number of valence electrons of chlorine is 7, and one bond is formed by two electrons in which one-one electrons are donated by each atom. The bonds must be formed in such a way that each atom can complete its octet.
There must be five dots around the phosphorus atom and there must be seven electrons around the chlorine atom.
In phosphorus pentachloride, there is one phosphorus atom and there are five chlorine atoms. So, the total electrons in the Lewis dot structure will be:
$5+7+7+7+7+7=40$
All the electrons of phosphorus are bonded to chlorine atom and the structure is given below:

Note:
If the overall charge on the molecule is negative then the electron is added to the molecule and if the overall charge on the molecule is positive then the electrons are deducted from the molecule. As the charge increases the number of electrons gained or lost also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




