
How do you draw the Lewis structure of \[I{{F}_{4}}^{-}\] ?
Answer
558.6k+ views
Hint:In order to achieve representation of ions used in Lewis Structure we should know about the Lewis Dot Diagram and elements Hybridization along with it. The major concept behind Lewis Structure understands how valence electrons interaction takes place.
Complete answer:
Here are the following steps that we need to consider while drawing Lewis Structure:
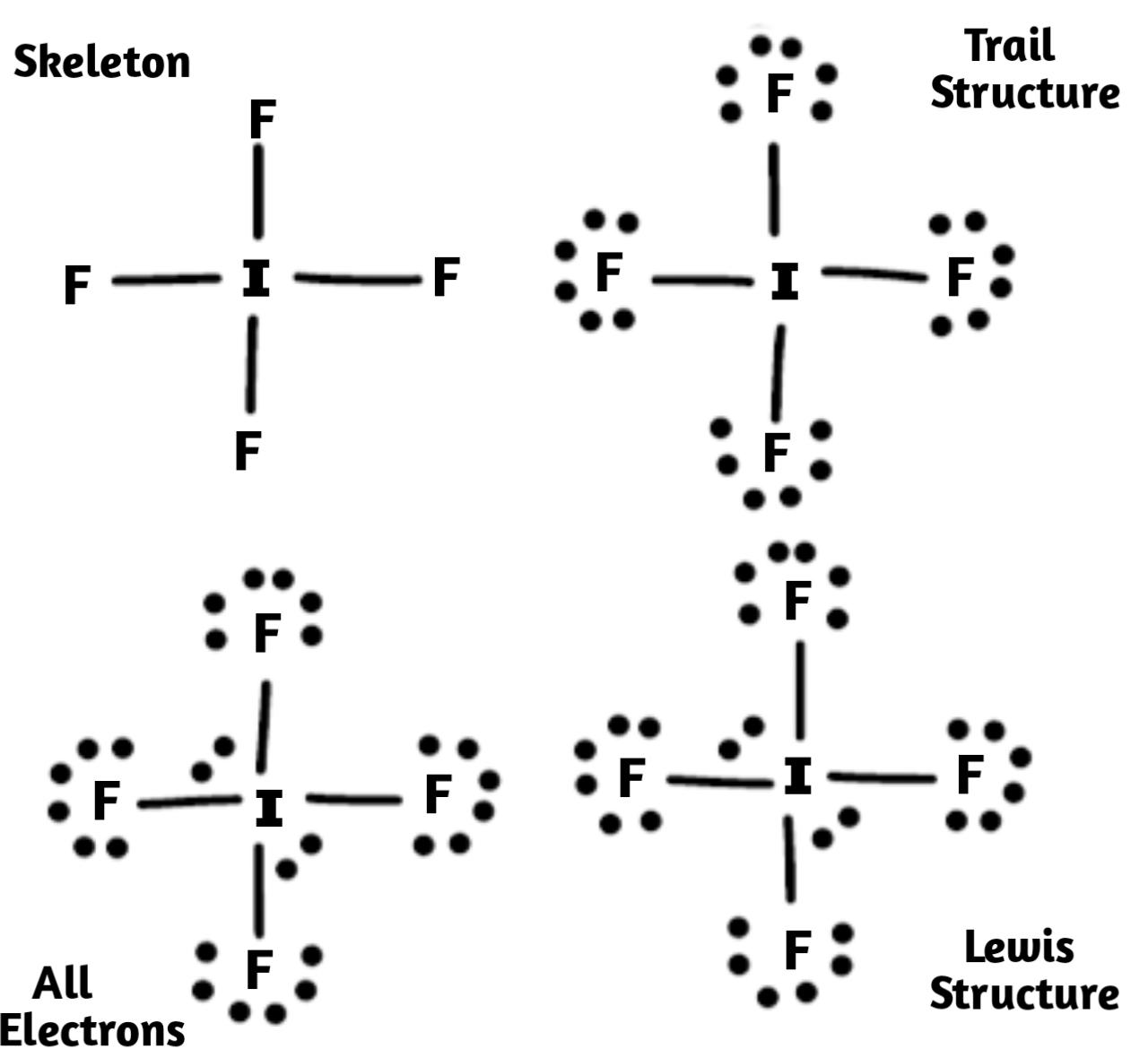
1) Firstly we have decided the central atom in the structure, and that we can identify with normally being the least electronegative atom that is Iodine here.
2) Then we need to draw a skeleton structure in which that other atom are single bonded to the central atom iodine:
3) After we have drawn the skeletal structure we have to draw the trial structure by putting all electrons pairs around each and every atom until each gets a complete octet.
4) After that we need to count the valence electrons in the compound trial structure in total they are $32$ .
5) Now we had to count the valence electrons that we actually have available in compound which is given by \[1I\text{ }+\text{ }4F\text{ }+\text{ }1e-\text{ }=\text{ }\left( 1\times 7 \right)+\left( 4\times 7 \right)+\left( 1 \right)\text{ }=\text{ }7+28+1\text{ }=\text{ }36\] this clears that the trial structure has four electrons extra in total.
6) Now we have to keep adding these extra electrons to the central atom and the structure will be called "all Electrons".
7) Lastly we have to calculate the formal charge on each atom. The Iodine atom has a charge of $-1$ . We place these charges on the iodine atom, and we get the required Lewis structure of \[I{{F}_{4}}^{-}\] .
Note:
1) We should always remember that electrons exist outside of an atom‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
2) Also the outermost principal energy level that contains electrons is called the valence level and contains valence electrons.
3) Lewis structures are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs which we should keep in mind before solving it.
4) Lewis structure does not visualize the electrons in the inner principal energy levels.
Complete answer:
Here are the following steps that we need to consider while drawing Lewis Structure:
1) Firstly we have decided the central atom in the structure, and that we can identify with normally being the least electronegative atom that is Iodine here.
2) Then we need to draw a skeleton structure in which that other atom are single bonded to the central atom iodine:
3) After we have drawn the skeletal structure we have to draw the trial structure by putting all electrons pairs around each and every atom until each gets a complete octet.
4) After that we need to count the valence electrons in the compound trial structure in total they are $32$ .
5) Now we had to count the valence electrons that we actually have available in compound which is given by \[1I\text{ }+\text{ }4F\text{ }+\text{ }1e-\text{ }=\text{ }\left( 1\times 7 \right)+\left( 4\times 7 \right)+\left( 1 \right)\text{ }=\text{ }7+28+1\text{ }=\text{ }36\] this clears that the trial structure has four electrons extra in total.
6) Now we have to keep adding these extra electrons to the central atom and the structure will be called "all Electrons".
7) Lastly we have to calculate the formal charge on each atom. The Iodine atom has a charge of $-1$ . We place these charges on the iodine atom, and we get the required Lewis structure of \[I{{F}_{4}}^{-}\] .

Note:
1) We should always remember that electrons exist outside of an atom‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
2) Also the outermost principal energy level that contains electrons is called the valence level and contains valence electrons.
3) Lewis structures are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs which we should keep in mind before solving it.
4) Lewis structure does not visualize the electrons in the inner principal energy levels.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




