
Draw the Lewis structure for \({\rm{CO}}_{\rm{3}}^{{\rm{2}} - }\) .
Answer
586.2k+ views
Hint: We know that, according to Kossel and Lewis atoms of different elements take part in chemical combination in order to complete their octet (to have eight electrons in valence shell) or duplet (to have two valence electrons in case of hydrogen, helium etc. This is known as the octet rule.
Complete step by step answer: We know that Lewis structures are those structures in which shared electrons are represented in dots.
Let’s discuss various steps of writing Lewis structure for simple molecules or ions.
-First we have to count the number of valence electrons of the atoms involved in a particular molecule or ion and add them.
-If the species is cation, we have to subtract the number of electrons corresponding to the positive charges from the total.
-If the species is anion, we have to add the number of electrons corresponding to the negative charges into the total.
-Next we have to select the central atom which is generally least electronegative in nature. Now, we have to write the skeletal structure on the basis of intelligent guesses. We must remember that monatomic atoms like H, Cl, F etc. always occupy terminal positions.
-Then, we have to place one shared electron pair between every pair of atoms to represent a single bond. The remaining electrons may account for either multiple bonds or they may act as lone pairs.
Let’s come to the question. We have to draw the Lewis structure of \({\rm{CO}}_{\rm{3}}^{{\rm{2}} - }\).
First we count the number of valence electrons of the atoms.
Valence electrons\( = 4 + 3 \times 6 = 22\)
As the species bears -2 charge, 2 electrons are to be added. So, the total number of valence electrons is 24.
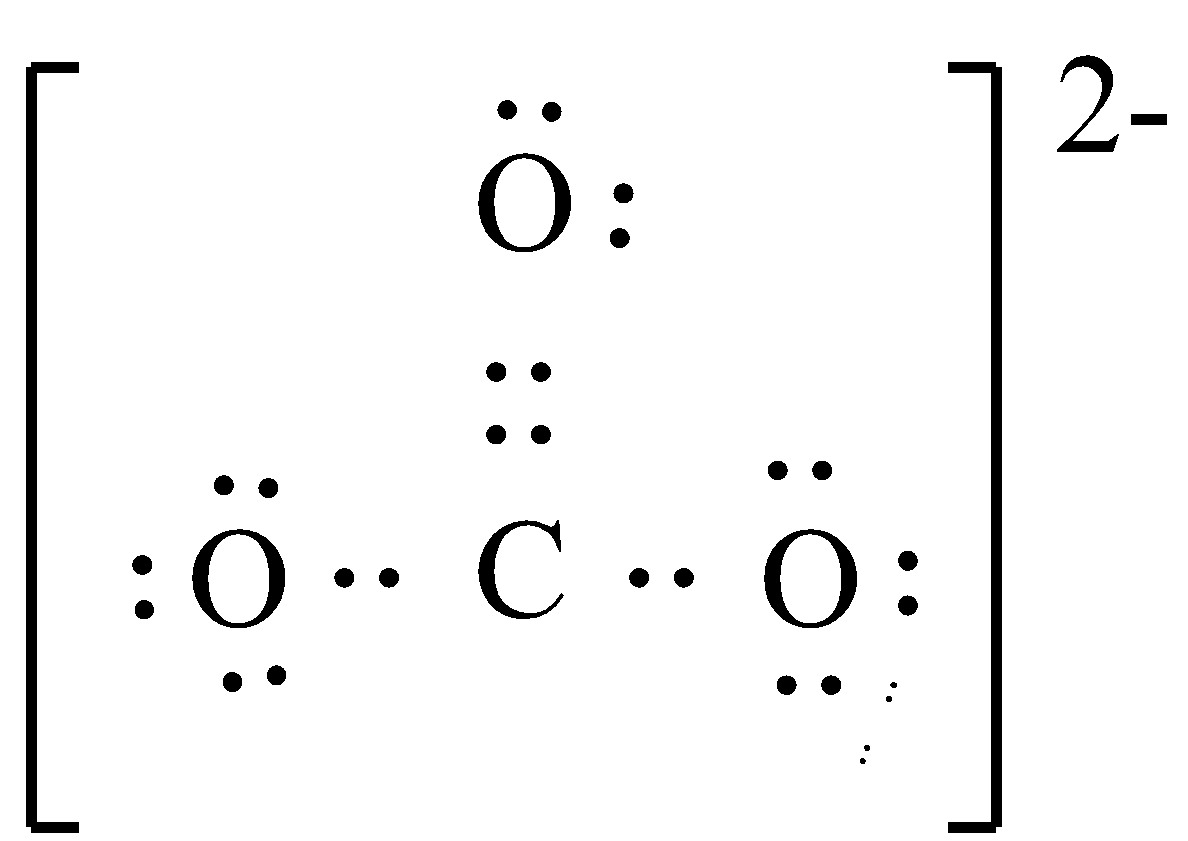
Now, we have to draw the skeletal structure.

Now, we have to place one shared pair of electrons between all the three pairs of electrons to complete their octet. In order to obtain an octet of C atoms, we have to add one more electron pair between the carbon and oxygen atom.
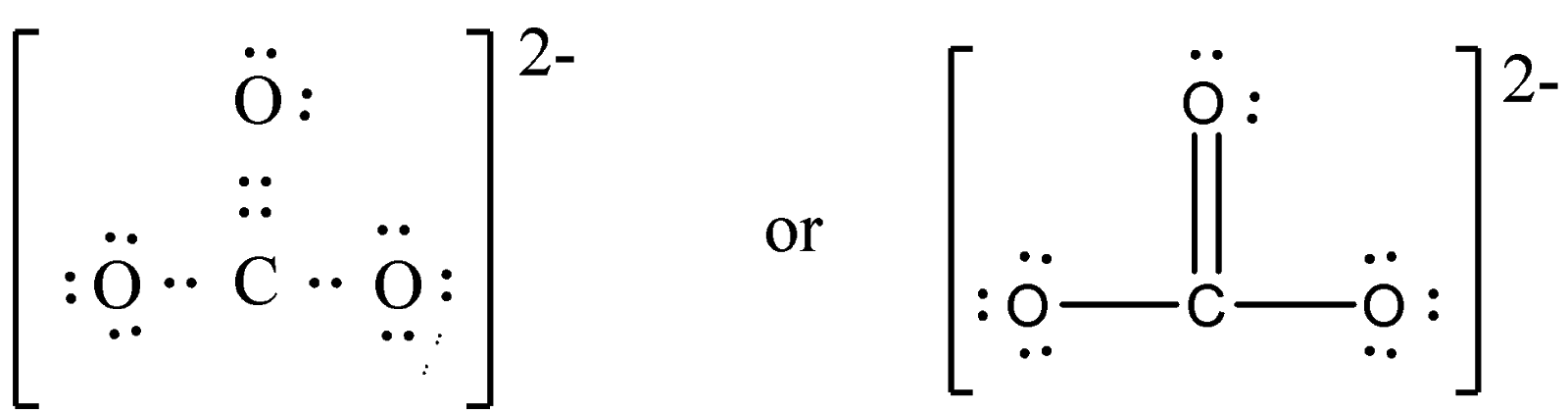
From the above structure we clearly see that 24 electrons (12 pairs) are present in the molecule. Hence, Lewis structure of \({\rm{CO}}_{\rm{3}}^{{\rm{2}} - }\) is,
Note: It is to be noted that octet rule is given keeping in view the inert character of noble gas elements. However, some of the elements, especially xenon has formed compounds with fluorine and oxygen (\(Xe{F_2}\), \[{\rm{XeO}}{{\rm{F}}_{\rm{4}}}\] etc.) his shows that xenon undergoes chemical combination in spite of the presence of complete octet. Therefore, the significance of the octet rule was challenged.
Complete step by step answer: We know that Lewis structures are those structures in which shared electrons are represented in dots.
Let’s discuss various steps of writing Lewis structure for simple molecules or ions.
-First we have to count the number of valence electrons of the atoms involved in a particular molecule or ion and add them.
-If the species is cation, we have to subtract the number of electrons corresponding to the positive charges from the total.
-If the species is anion, we have to add the number of electrons corresponding to the negative charges into the total.
-Next we have to select the central atom which is generally least electronegative in nature. Now, we have to write the skeletal structure on the basis of intelligent guesses. We must remember that monatomic atoms like H, Cl, F etc. always occupy terminal positions.
-Then, we have to place one shared electron pair between every pair of atoms to represent a single bond. The remaining electrons may account for either multiple bonds or they may act as lone pairs.
Let’s come to the question. We have to draw the Lewis structure of \({\rm{CO}}_{\rm{3}}^{{\rm{2}} - }\).
First we count the number of valence electrons of the atoms.
Valence electrons\( = 4 + 3 \times 6 = 22\)
As the species bears -2 charge, 2 electrons are to be added. So, the total number of valence electrons is 24.
Now, we have to draw the skeletal structure.

Now, we have to place one shared pair of electrons between all the three pairs of electrons to complete their octet. In order to obtain an octet of C atoms, we have to add one more electron pair between the carbon and oxygen atom.
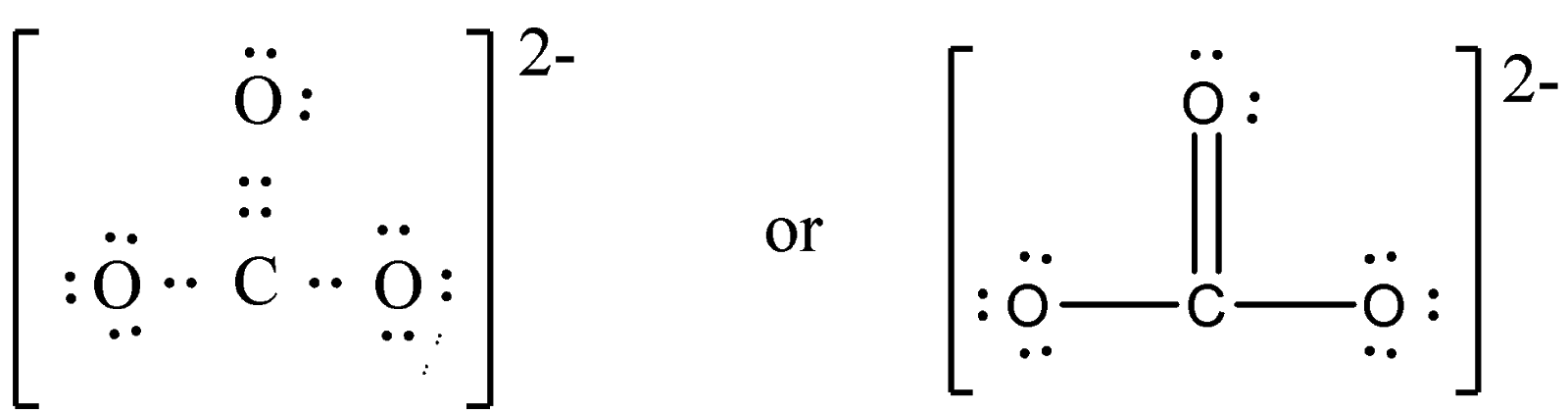
From the above structure we clearly see that 24 electrons (12 pairs) are present in the molecule. Hence, Lewis structure of \({\rm{CO}}_{\rm{3}}^{{\rm{2}} - }\) is,

Note: It is to be noted that octet rule is given keeping in view the inert character of noble gas elements. However, some of the elements, especially xenon has formed compounds with fluorine and oxygen (\(Xe{F_2}\), \[{\rm{XeO}}{{\rm{F}}_{\rm{4}}}\] etc.) his shows that xenon undergoes chemical combination in spite of the presence of complete octet. Therefore, the significance of the octet rule was challenged.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




