
How can I draw the Lewis structure for $CO$?
Answer
564.6k+ views
Hint: $CO$ is known by the name carbon monoxide which is a colorless, odorless and tasteless flammable gas. Its density is slightly less than the air. It consists of one carbon atom and one oxygen atom and it is somewhat toxic to animals that use hemoglobin as an oxygen carrier.
Complete answer:
Lewis structure basically tells us how the electrons are paired. In this every dot represents an electron and pair of dots between chemical symbols for atoms represents the bond. The main steps of draw a Lewis structure are as follows:
1. Carbon monoxide consists of two atoms known as carbon and oxygen.
2. Oxygen atoms have atomic number 8 and valence electrons i.e. outermost electrons are 6 in this case which forms 2 lone pairs. Carbon atoms have atomic number 6 and the valence electrons present in carbon atoms are 4 which consider that no lone pairs are formed in carbon.
3. This valency is satisfied by donation of lone pair of electrons for bonding by the oxygen atom.
4. This shows a triple bond connecting carbon and oxygen atoms with each other in which each holding a lone pair of electrons.
Hence on the basis of above discussion we can say that the Lewis structure of $CO$ can be drawn as:
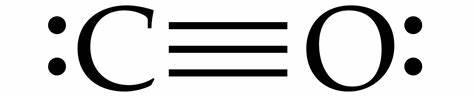
Note:
If we know the molecular formula of any compound we can easily draw its Lewis dot structure which is also known by the name electron dot structure. These diagrams generally describe the chemical bonding between atoms in a molecule and also display the total number of lone pairs present in each of the atoms.
Complete answer:
Lewis structure basically tells us how the electrons are paired. In this every dot represents an electron and pair of dots between chemical symbols for atoms represents the bond. The main steps of draw a Lewis structure are as follows:
1. Carbon monoxide consists of two atoms known as carbon and oxygen.
2. Oxygen atoms have atomic number 8 and valence electrons i.e. outermost electrons are 6 in this case which forms 2 lone pairs. Carbon atoms have atomic number 6 and the valence electrons present in carbon atoms are 4 which consider that no lone pairs are formed in carbon.
3. This valency is satisfied by donation of lone pair of electrons for bonding by the oxygen atom.
4. This shows a triple bond connecting carbon and oxygen atoms with each other in which each holding a lone pair of electrons.
Hence on the basis of above discussion we can say that the Lewis structure of $CO$ can be drawn as:
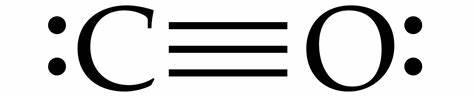
Note:
If we know the molecular formula of any compound we can easily draw its Lewis dot structure which is also known by the name electron dot structure. These diagrams generally describe the chemical bonding between atoms in a molecule and also display the total number of lone pairs present in each of the atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




