
How would you draw the Lewis resonance structure of $C{H_2}Br$?
Answer
557.1k+ views
Hint: The compound $C{H_2}Br$ is a radical named as bromomethyl radical. The bromomethyl radical does not contain any charge, any double bond. It does not even contain any central atom. The Lewis structure is the representation of the valence electron in a molecule. When the molecule cannot be described by one Lewis structure, then resonance structures are formed which is a set of two or more Lewis structures.
Complete step by step answer:
The given compound is $C{H_2}Br$, it is a bromomethyl radical.
When the structure of the molecule cannot be represented by one Lewis structure, resonance effect is seen. The resonance structure is the set of two or more Lewis structures that describes the electronic bonding of single polyatomic species.
The bromomethyl radical has only one possible Lewis structure, it does not show any resonance structure. The molecule contains odd number of electrons
The structure of bromomethyl radical is shown below.
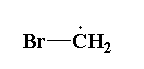
In bromo methyl radical $C{H_2}Br$, one carbon atom is present, two hydrogen atoms are present and one bromine atom is present.
The valence electrons of carbon is 4.
The valence electrons of hydrogen is ($1 \times 2$)=2
The valence electrons of bromine is 7
The total electrons are 13.
As the radical contains no charge, no double bonds and bromine is not the central atom, there are no resonance structures formed. Only one Lewis structure is formed.
Note:
The Lewis structure does not explain the geometry of the compound. In the resonance form of the compound, shifting of electrons takes place and not shifting of atoms takes place.
Complete step by step answer:
The given compound is $C{H_2}Br$, it is a bromomethyl radical.
When the structure of the molecule cannot be represented by one Lewis structure, resonance effect is seen. The resonance structure is the set of two or more Lewis structures that describes the electronic bonding of single polyatomic species.
The bromomethyl radical has only one possible Lewis structure, it does not show any resonance structure. The molecule contains odd number of electrons
The structure of bromomethyl radical is shown below.
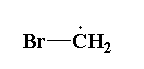
In bromo methyl radical $C{H_2}Br$, one carbon atom is present, two hydrogen atoms are present and one bromine atom is present.
The valence electrons of carbon is 4.
The valence electrons of hydrogen is ($1 \times 2$)=2
The valence electrons of bromine is 7
The total electrons are 13.
As the radical contains no charge, no double bonds and bromine is not the central atom, there are no resonance structures formed. Only one Lewis structure is formed.
Note:
The Lewis structure does not explain the geometry of the compound. In the resonance form of the compound, shifting of electrons takes place and not shifting of atoms takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




