
Draw the Lewis dot structure of the following.
$C{O_3}^{2 - }$, $HCl{O_4}$, $HN{O_3}$
Answer
513.3k+ views
Hint: We know that Lewis dot structures mirror the electronic designs of the components, including how the electrons are matched. Lewis structures are a helpful method to sum up certain data about holding and might be considered as "electron accounting". In Lewis dot structures each dab addresses an electron. A couple of dots between compound symbols for atoms address a bond.
Complete answer:
As we know, Lewis symbols are graphs that address the valence electrons of a particle. Lewis structures are graphs that address the valence electrons of iotas inside a particle. These Lewis symbols and Lewis structures help imagine the valence electrons of iotas and particles, regardless of whether they exist as solitary sets or inside bonds.
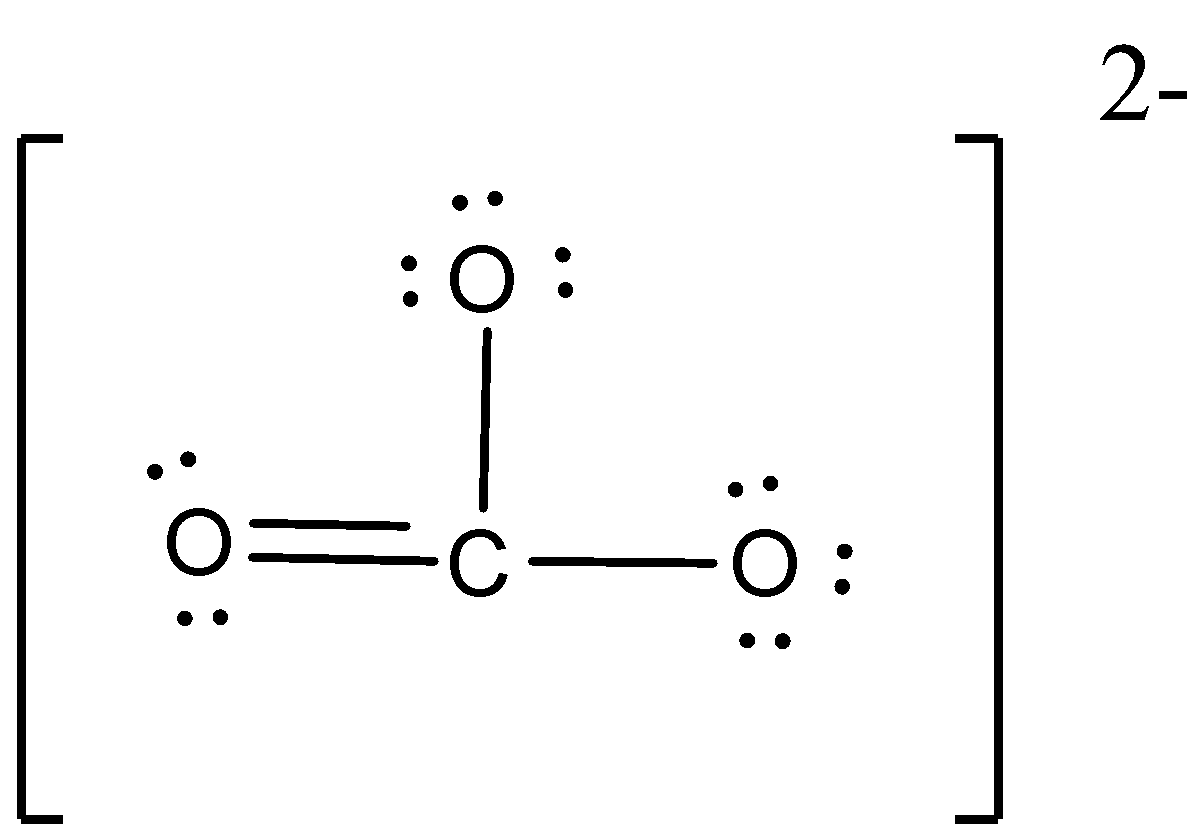
The Lewis structure of $C{O_3}^{2 - }$ is,
The Lewis structure of $HCl{O_4}$ is,

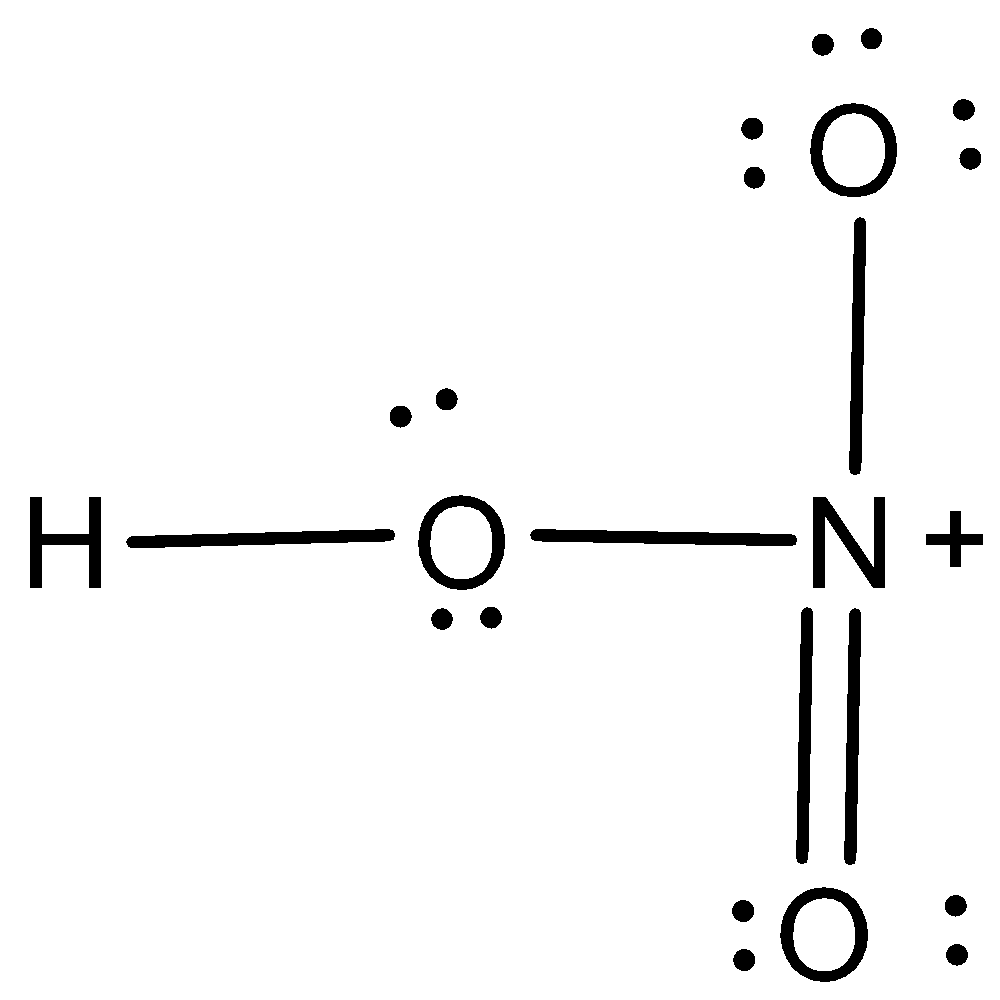
The Lewis structure of $HN{O_3}$ is,
Note:
We have to know that Lewis structures for polyatomic particles might be drawn by a similar technique. When tallying electrons, negative particles ought to have additional electrons set in their Lewis structures; positive particles ought to have fewer electrons than an uncharged atom. At the point when the Lewis design of a particle is composed, the whole construction is set in sections, and the charge is composed as a superscript on the upper right, outside the sections.
An easier strategy has been proposed for building Lewis structures, taking out the requirement for electron checking: the iotas are drawn appearance the valence electrons; securities are then framed by matching up valence electrons of the particles associated with the security making interaction, and anions and cations are shaped by adding or eliminating electrons to/from the fitting molecules.
Complete answer:
As we know, Lewis symbols are graphs that address the valence electrons of a particle. Lewis structures are graphs that address the valence electrons of iotas inside a particle. These Lewis symbols and Lewis structures help imagine the valence electrons of iotas and particles, regardless of whether they exist as solitary sets or inside bonds.
The Lewis structure of $C{O_3}^{2 - }$ is,

The Lewis structure of $HCl{O_4}$ is,

The Lewis structure of $HN{O_3}$ is,

Note:
We have to know that Lewis structures for polyatomic particles might be drawn by a similar technique. When tallying electrons, negative particles ought to have additional electrons set in their Lewis structures; positive particles ought to have fewer electrons than an uncharged atom. At the point when the Lewis design of a particle is composed, the whole construction is set in sections, and the charge is composed as a superscript on the upper right, outside the sections.
An easier strategy has been proposed for building Lewis structures, taking out the requirement for electron checking: the iotas are drawn appearance the valence electrons; securities are then framed by matching up valence electrons of the particles associated with the security making interaction, and anions and cations are shaped by adding or eliminating electrons to/from the fitting molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




