
Draw the electron dot structure of ${{\rm{F}}_{\rm{2}}}$ (fluorine).
Answer
585k+ views
Hint: We know that the structure of the molecules in terms of the valence electrons present in the valence shell of each atom in the molecule is referred to as Lewis structures or electron dot structure.
Complete step by step answer:
We know that Lewis structures are drawn for molecules containing either covalent bonds or coordinate bonds. These structures are drawn by placing the atoms in an order that less electronegative atom or the atom with the highest valency is kept in the center and more electronegative atoms surround it.
In Lewis dot structure, each atom is then provided with their valence electrons and sharing of electrons is done to complete their octet or doublet between the central and surrounding atoms. However, the remaining electrons belonging to valence shells are kept on each atom wherever applicable which are known as lone pairs.
In ${{\rm{F}}_{\rm{2}}}$ molecule, fluorine has the atomic number $9$. Its electronic configuration is ${\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{5}}}$. The number of valence electrons present in fluorine is $7$ which are present in ${\rm{2s}}$ and ${\rm{2p}}$ orbitals. Fluorine requires only one valence electron in order to complete its octet. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{F}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. So let’s draw the electron dot structure of ${{\rm{F}}_{\rm{2}}}$ which is shown below.
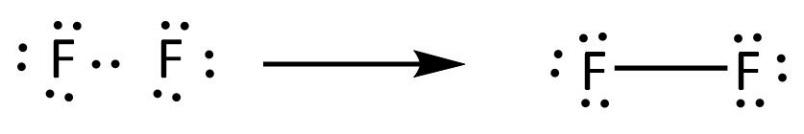
There is one bonded pair of electrons and three lone pairs of electrons on each fluorine atom.
Note:
The electron dot structures of molecules are very helpful in the determination of bonding and nonbonding electron pairs.
Complete step by step answer:
We know that Lewis structures are drawn for molecules containing either covalent bonds or coordinate bonds. These structures are drawn by placing the atoms in an order that less electronegative atom or the atom with the highest valency is kept in the center and more electronegative atoms surround it.
In Lewis dot structure, each atom is then provided with their valence electrons and sharing of electrons is done to complete their octet or doublet between the central and surrounding atoms. However, the remaining electrons belonging to valence shells are kept on each atom wherever applicable which are known as lone pairs.
In ${{\rm{F}}_{\rm{2}}}$ molecule, fluorine has the atomic number $9$. Its electronic configuration is ${\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{5}}}$. The number of valence electrons present in fluorine is $7$ which are present in ${\rm{2s}}$ and ${\rm{2p}}$ orbitals. Fluorine requires only one valence electron in order to complete its octet. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{F}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. So let’s draw the electron dot structure of ${{\rm{F}}_{\rm{2}}}$ which is shown below.

There is one bonded pair of electrons and three lone pairs of electrons on each fluorine atom.
Note:
The electron dot structures of molecules are very helpful in the determination of bonding and nonbonding electron pairs.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




