
Draw the electron dot structure of ${{H}_{2}}S$ (hydrogen sulphide)?
Answer
546k+ views
Hint: The hybridisation of the central atom sulphur in hydrogen sulphide is$s{{p}^{3}}$. The two hydrogen atoms are attached to the central atom sulphur by sigma bonds, and sulphur has two lone pairs of electrons.
Complete answer:
The Lewis structure of ${{H}_{2}}S$ is very similar to${{H}_{2}}O$. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for${{H}_{2}}S$ has a total of 8 valence electrons.
Hydrogen has 1 valence electron, but we have two Hydrogens here. So, the total valence electrons of hydrogen are 2. Sulphur is in group 16 on the periodic table, so it has 6 valence electrons. Therefore, the total valence electrons of hydrogen sulphide are eight. Now, two valence electrons of sulphur bond with two hydrogens and we have four electrons remaining on sulphur which make up two lone pairs of electrons of sulphur. The Lewis dot structure of hydrogen sulphide is given below:
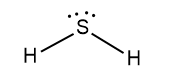
Hydrogen sulphide is a bent shaped molecule. Its molecular weight is 34g/mol. It is a colourless gas having a strong odour of rotten eggs. Its boiling point is$-{{60.2}^{\circ }}C$. It is a highly toxic and flammable gas. It is also heavier than air.
Note:
If the lone pair of electrons are not accounted for, a student might confuse the hybridization of ${{H}_{2}}S$ to be sp. The shape of the molecule in that case would come out to be linear.
Complete answer:
The Lewis structure of ${{H}_{2}}S$ is very similar to${{H}_{2}}O$. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for${{H}_{2}}S$ has a total of 8 valence electrons.
Hydrogen has 1 valence electron, but we have two Hydrogens here. So, the total valence electrons of hydrogen are 2. Sulphur is in group 16 on the periodic table, so it has 6 valence electrons. Therefore, the total valence electrons of hydrogen sulphide are eight. Now, two valence electrons of sulphur bond with two hydrogens and we have four electrons remaining on sulphur which make up two lone pairs of electrons of sulphur. The Lewis dot structure of hydrogen sulphide is given below:

Hydrogen sulphide is a bent shaped molecule. Its molecular weight is 34g/mol. It is a colourless gas having a strong odour of rotten eggs. Its boiling point is$-{{60.2}^{\circ }}C$. It is a highly toxic and flammable gas. It is also heavier than air.
Note:
If the lone pair of electrons are not accounted for, a student might confuse the hybridization of ${{H}_{2}}S$ to be sp. The shape of the molecule in that case would come out to be linear.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




