
Draw the electron dot diagram to show the formation of a stable positive ion from a molecule having two lone pairs of electrons and another atom short of a lone pair of electrons [e.g. \[{H^{1 + }}\]].
Answer
478.2k+ views
Hint: We first need to know what an electron dot diagram is and accordingly draw one such diagram for a stable positive ion from a molecule having two lone pairs of electrons and another atom short of a lone pair of electrons. A lone pair is a pair of valence electrons in a covalent bond that is not shared with another atom.
Complete answer:
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures, are diagrams that depict the bonding between atoms in a molecule, as well as any lone pairs of electrons that may be present. Lewis structures add lines between atoms to indicate shared pairs in a chemical bond, extending the notion of the electron dot diagram. The sum of the valence electrons on each atom equals the total number of electrons represented in a Lewis structure. Lewis structures do not include non-valence electrons. The Lewis structure of an ion is enclosed in brackets, and the charge is represented as a superscript outside the brackets on the upper right.
The one such example asked in the question is the formation of hydronium ions. The reaction is as follows:
\[{H_2}O + {H^ + } \to {H_3}{O^ + }\]
In the reaction above, ${H_3}{O^ + }$ is the stable positive hydronium ion which has resulted from water, ${H_2}O$ which has two lone pairs of electrons and ${H^ + }$ atom short of a lone pair of electrons. The electron dot diagrams are given as follows:
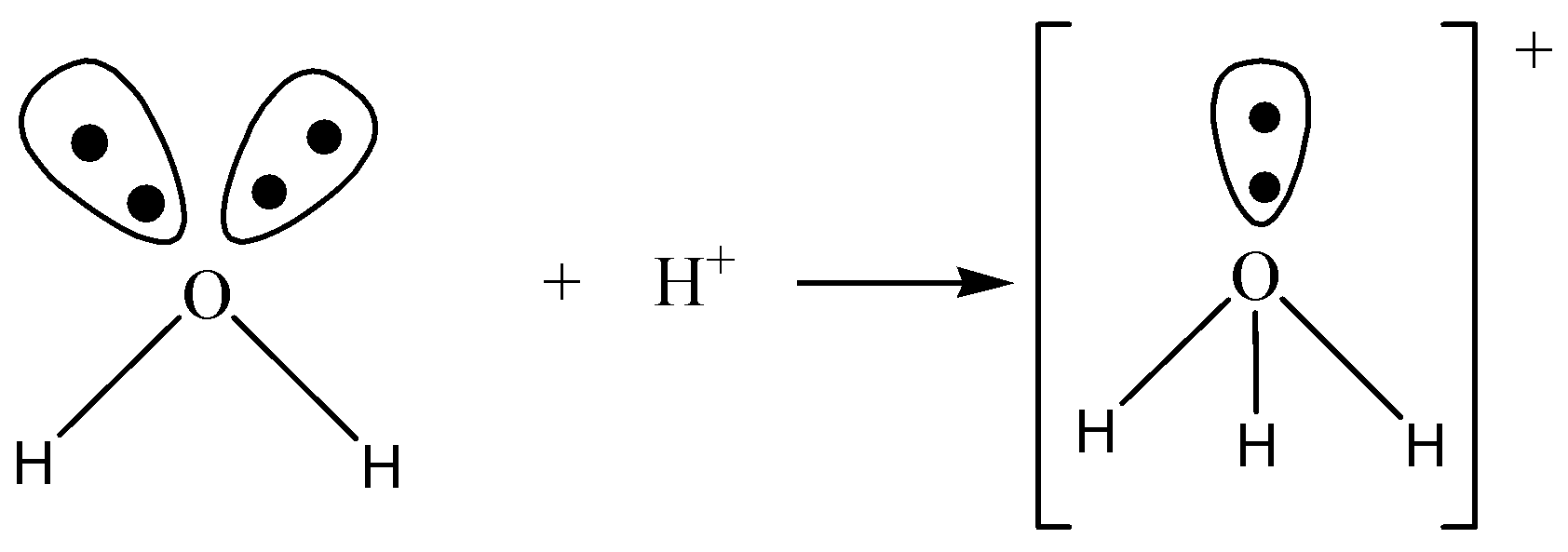
Electron dot diagrams of water and hydronium ions.
Note:
Any covalently bound molecule, as well as coordination compounds, can be represented by a Lewis structure but it is worth noting that there are several basic and archetypal molecular systems for which a Lewis description is misleading or erroneous, at least in its original form. Notably, erroneous bond ordering, bond lengths, and/or magnetic characteristics are inferred from naïve Lewis structure drawings for compounds confirmed to have unpaired electrons empirically. The phenomena of aromaticity are likewise not explained by a simple Lewis model.
Complete answer:
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures, are diagrams that depict the bonding between atoms in a molecule, as well as any lone pairs of electrons that may be present. Lewis structures add lines between atoms to indicate shared pairs in a chemical bond, extending the notion of the electron dot diagram. The sum of the valence electrons on each atom equals the total number of electrons represented in a Lewis structure. Lewis structures do not include non-valence electrons. The Lewis structure of an ion is enclosed in brackets, and the charge is represented as a superscript outside the brackets on the upper right.
The one such example asked in the question is the formation of hydronium ions. The reaction is as follows:
\[{H_2}O + {H^ + } \to {H_3}{O^ + }\]
In the reaction above, ${H_3}{O^ + }$ is the stable positive hydronium ion which has resulted from water, ${H_2}O$ which has two lone pairs of electrons and ${H^ + }$ atom short of a lone pair of electrons. The electron dot diagrams are given as follows:

Electron dot diagrams of water and hydronium ions.
Note:
Any covalently bound molecule, as well as coordination compounds, can be represented by a Lewis structure but it is worth noting that there are several basic and archetypal molecular systems for which a Lewis description is misleading or erroneous, at least in its original form. Notably, erroneous bond ordering, bond lengths, and/or magnetic characteristics are inferred from naïve Lewis structure drawings for compounds confirmed to have unpaired electrons empirically. The phenomena of aromaticity are likewise not explained by a simple Lewis model.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




