
Draw the electron dot/ atomic orbital structure to show the formation of the following compounds: [Atomic number: Ca = 20, O = 8, C = 6, Cl = 17]
i.) $CaO$
ii.) $CC{{l}_{4}}$
Answer
566.4k+ views
Hint: Lewis dot structures also known as Lewis electron-dot formulas. It uses dots arranged around the chemical symbol for an element to represent the valence electron configuration of the atoms in the element.
Complete Solution :
Given in the question:
The atomic number of calcium i.e. Ca = 20
The electronic configuration of Ca = 2,8,8,2
The Lewis dot structure of Calcium will have 2 electrons and it is represented as:

The atomic number of oxygen i.e. O = 8
The electronic configuration of O = 2,6
- The Lewis dot structure of O will have 6 electrons and it is represented as:

- The formation of CaO will be represented as:
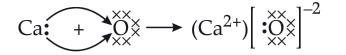
Now,
The atomic number of chlorine i.e. Cl = 17
The electronic configuration of Cl = 2,8,7
- The Lewis dot structure of Cl will have 7 electrons and it is represented as:

The atomic number of carbon i.e. C = 6
The electronic configuration of C = 2,4
- The Lewis dot structure of C will have 4 electrons and it is represented as:

- The formation of $CC{{l}_{4}}$ will be represented as:
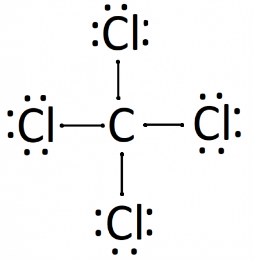
Note: The possibility to make a mistake is that while drawing the Lewis dot structure of an atom then you may miscount the valence electrons due to which double or triple bonds are drawn incorrectly. Also, don’t forget the lone pairs of an atom.
Complete Solution :
Given in the question:
The atomic number of calcium i.e. Ca = 20
The electronic configuration of Ca = 2,8,8,2
The Lewis dot structure of Calcium will have 2 electrons and it is represented as:

The atomic number of oxygen i.e. O = 8
The electronic configuration of O = 2,6
- The Lewis dot structure of O will have 6 electrons and it is represented as:

- The formation of CaO will be represented as:
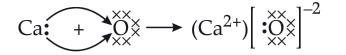
Now,
The atomic number of chlorine i.e. Cl = 17
The electronic configuration of Cl = 2,8,7
- The Lewis dot structure of Cl will have 7 electrons and it is represented as:

The atomic number of carbon i.e. C = 6
The electronic configuration of C = 2,4
- The Lewis dot structure of C will have 4 electrons and it is represented as:

- The formation of $CC{{l}_{4}}$ will be represented as:

Note: The possibility to make a mistake is that while drawing the Lewis dot structure of an atom then you may miscount the valence electrons due to which double or triple bonds are drawn incorrectly. Also, don’t forget the lone pairs of an atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




