
How do you draw the electron configuration diagram for manganese?
Answer
564.9k+ views
Hint: Manganese is the element of group 7 of the d-block and its atomic number is 25 so, there are 25 electrons in the atom. Arrange these 25 electrons in the increasing order of the energy of the shell, since it is a d-block element, its last electron should come in the d-subshell. Now form a chart having all subshells and fill them accordingly.
Complete answer:
There are various shells in the atoms which are denoted by K, L, M, N, etc, which represents the specific energy of the shell. The shell K represents 1, the shell L represents 2, the shell M represents 3, the shell N represents 4. Each shell has specific sub-shells which are s, p, d, f, etc.
The K shell has one sub-shell (s), the L shell has two sub-shells (s, p), the M shell has three sub-shells (s, p, d), the N shell has four sub-shells (s, p, d, f).
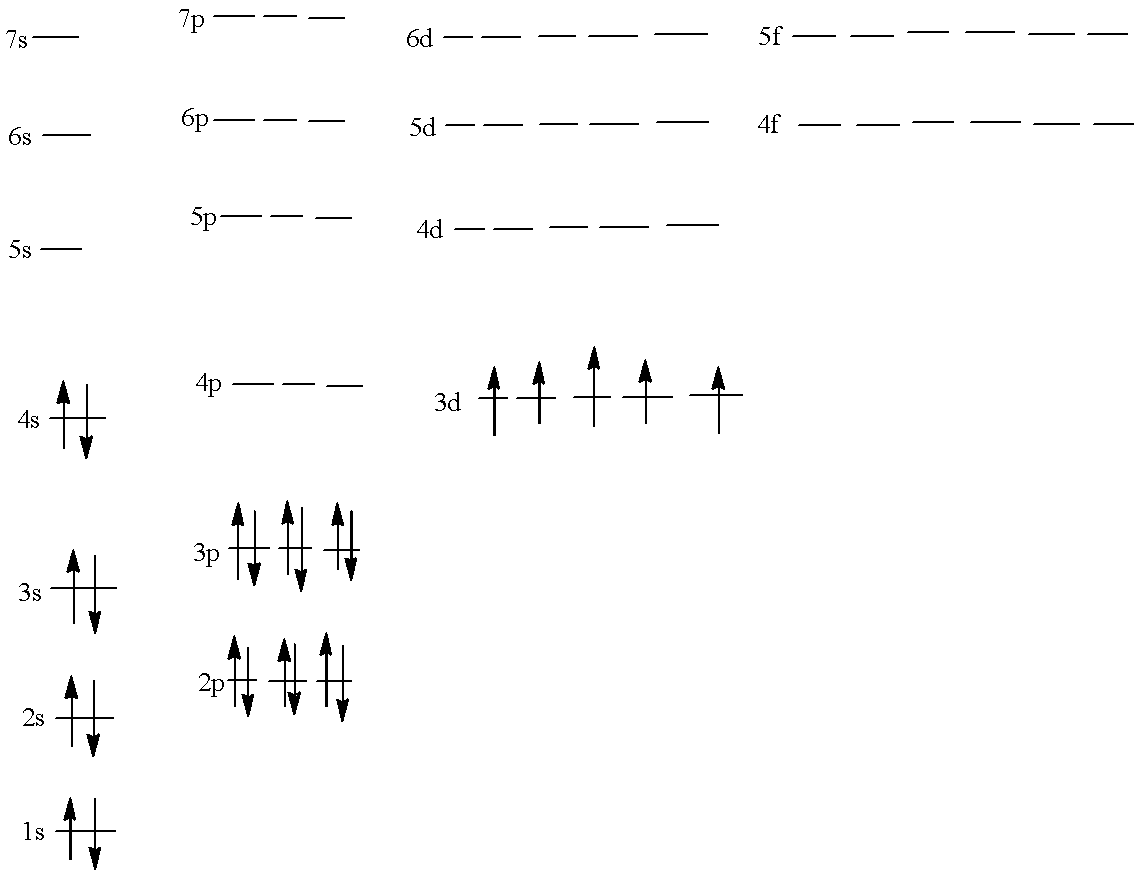
Manganese is the element of group 7 of the d-block and its atomic number is 25 so, there are 25 electrons in the atom. Arrange these 25 electrons in the increasing order of the energy of the shell, since it is a d-block element, its last electron should come in the d-subshell.
The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}}$
Now, forming the chart in which all the subshells are present and the electrons are filled according to the configuration given above:
Note:
It must be noted that in the 3-d sub-shell all the orbitals have only one electron because the pairing cannot be done; all the orbitals get one electron each. This is the basis of Hund’s rule.
Complete answer:
There are various shells in the atoms which are denoted by K, L, M, N, etc, which represents the specific energy of the shell. The shell K represents 1, the shell L represents 2, the shell M represents 3, the shell N represents 4. Each shell has specific sub-shells which are s, p, d, f, etc.
The K shell has one sub-shell (s), the L shell has two sub-shells (s, p), the M shell has three sub-shells (s, p, d), the N shell has four sub-shells (s, p, d, f).
Manganese is the element of group 7 of the d-block and its atomic number is 25 so, there are 25 electrons in the atom. Arrange these 25 electrons in the increasing order of the energy of the shell, since it is a d-block element, its last electron should come in the d-subshell.
The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}}$
Now, forming the chart in which all the subshells are present and the electrons are filled according to the configuration given above:

Note:
It must be noted that in the 3-d sub-shell all the orbitals have only one electron because the pairing cannot be done; all the orbitals get one electron each. This is the basis of Hund’s rule.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




