
Draw the diagram of the blast furnace used in the extraction of iron and label the molten iron.
Answer
595.5k+ views
Hint: We have to draw the diagram of the blast furnace used in the extraction of iron. It is used for the common ores like iron oxides to extract the iron with the help of reduction with carbon. The apparatus consists of many parts like hot air blast, tap hole etc. Design the blast furnace.
Complete step by step answer:
First, let us know about the use of blast furnaces. As mentioned it is used to attain the iron by reduction with the carbon, and carbon is attained in the form of coke. The ores extracted in the blast furnace are hematite, magnetite.
Now, we will draw the diagram of a blast furnace used in the extraction of iron.
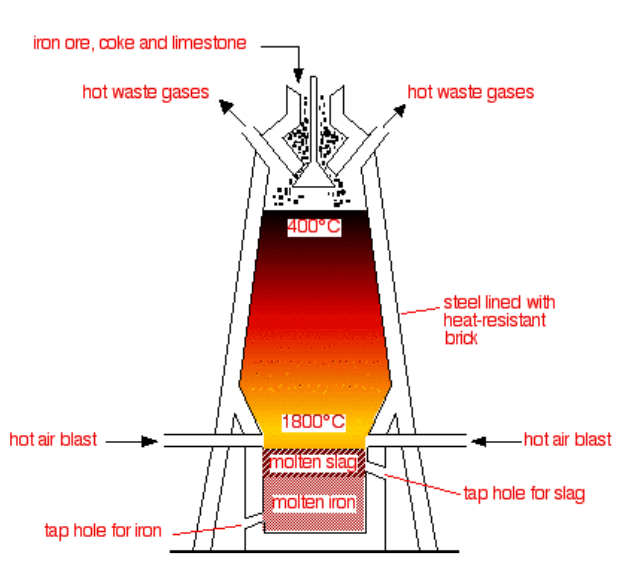
If we talk about the main purpose of the blast furnace, then it reduces the metal to its liquid state.
Talking about the composition of blast furnaces it is composed of steel making the differentiation with the bricks.
Now, we can see in the diagram that carbon forms coke, iron ore, and limestone is being discarded from the top, and from the bottom there is an entrance of hot air blast.
In the diagram there is the presence of a hopper, and the three elements discarded are crushed.
As we know, the coke is burnt so that temperature is yielded up to 2200 K.
Further proceeding the reactions will go on with the hot air blown from the bottom, and at last molten iron will be at the bottom as shown.
In the last we can conclude that the main purpose in the blast furnace is of hot air blast, and molten iron is labelled at the bottom representing the liquid state of iron.
Note: Don’t get confused between the molten slag, and molten iron. Molten iron is the liquid state attained of iron as mentioned; whereas molten slag is a type of impurity found in the molten iron. It is separated with the help of limestone.
Complete step by step answer:
First, let us know about the use of blast furnaces. As mentioned it is used to attain the iron by reduction with the carbon, and carbon is attained in the form of coke. The ores extracted in the blast furnace are hematite, magnetite.
Now, we will draw the diagram of a blast furnace used in the extraction of iron.

If we talk about the main purpose of the blast furnace, then it reduces the metal to its liquid state.
Talking about the composition of blast furnaces it is composed of steel making the differentiation with the bricks.
Now, we can see in the diagram that carbon forms coke, iron ore, and limestone is being discarded from the top, and from the bottom there is an entrance of hot air blast.
In the diagram there is the presence of a hopper, and the three elements discarded are crushed.
As we know, the coke is burnt so that temperature is yielded up to 2200 K.
Further proceeding the reactions will go on with the hot air blown from the bottom, and at last molten iron will be at the bottom as shown.
In the last we can conclude that the main purpose in the blast furnace is of hot air blast, and molten iron is labelled at the bottom representing the liquid state of iron.
Note: Don’t get confused between the molten slag, and molten iron. Molten iron is the liquid state attained of iron as mentioned; whereas molten slag is a type of impurity found in the molten iron. It is separated with the help of limestone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




