
Draw the conjugate acid of $N{{H}_{3}}$.
Answer
588k+ views
Hint: Ammonia has a lone pair of electrons on the nitrogen atom. It will act as a Lewis base and not as a Bronsted base. The conjugate acid structure of ammonia will not have the lone pair, instead it will have a hydrogen ion attached extra to the nitrogen atom.
Complete step by step answer:
Ammonia is a compound consisting of nitrogen atoms and has a formula $N{{H}_{3}}$. It is a stable hydride at room temperature and is one of the simplest pnictogen hydrides. The elements of group 15 are called pnictogens as they are highly toxic in nature.
Ammonia is a colourless gas with a distinct pungent smell. It is a common component of nitrogenous waste especially among aquatic organisms. It acts as a precursor for fertilisers and food as it fulfils the requirements of terrestrial organisms and plants respectively.
We will draw the structure of ammonia to understand the bonds and lone pairs present on the central nitrogen atom.

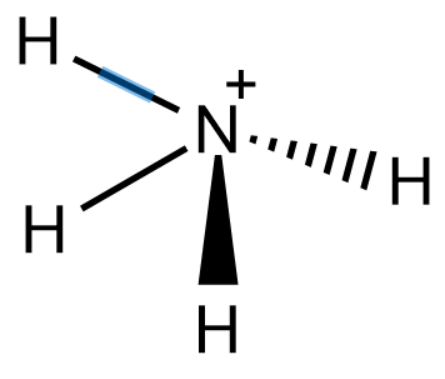
Nitrogen has a lone pair of electrons and thus acts as a Lewis base. The conjugate acid of $N{{H}_{3}}$ thus becomes
Additional Information:
Ammonia along with the uses mentioned above is a major component of various pharmaceutical products and commercial cleaning products. Nitrogen is mainly obtained by downward displacement of air and water.
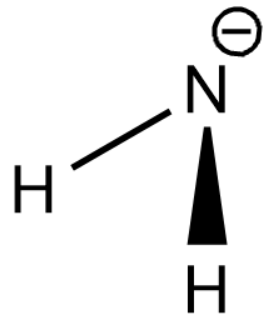
Note: The structure of $N{{H}_{3}}$ shows 3 replaceable hydrogen atoms attached to nitrogen. Thus $N{{H}_{3}}$ can act as Lewis acid as well and release a hydrogen atom as an ion. The structure of conjugate base of $N{{H}_{3}}$ is given below,
Complete step by step answer:
Ammonia is a compound consisting of nitrogen atoms and has a formula $N{{H}_{3}}$. It is a stable hydride at room temperature and is one of the simplest pnictogen hydrides. The elements of group 15 are called pnictogens as they are highly toxic in nature.
Ammonia is a colourless gas with a distinct pungent smell. It is a common component of nitrogenous waste especially among aquatic organisms. It acts as a precursor for fertilisers and food as it fulfils the requirements of terrestrial organisms and plants respectively.
We will draw the structure of ammonia to understand the bonds and lone pairs present on the central nitrogen atom.

Nitrogen has a lone pair of electrons and thus acts as a Lewis base. The conjugate acid of $N{{H}_{3}}$ thus becomes

Additional Information:
Ammonia along with the uses mentioned above is a major component of various pharmaceutical products and commercial cleaning products. Nitrogen is mainly obtained by downward displacement of air and water.
Note: The structure of $N{{H}_{3}}$ shows 3 replaceable hydrogen atoms attached to nitrogen. Thus $N{{H}_{3}}$ can act as Lewis acid as well and release a hydrogen atom as an ion. The structure of conjugate base of $N{{H}_{3}}$ is given below,

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




