
Draw the complete structure of the given compound (using hydrogen-carbon bonds).
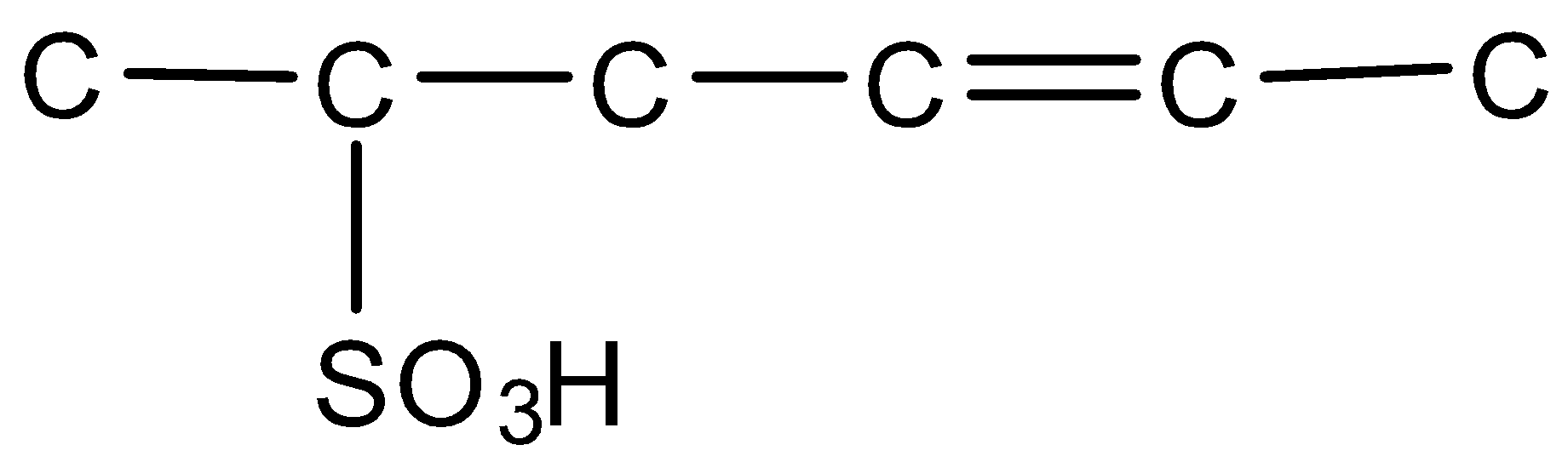
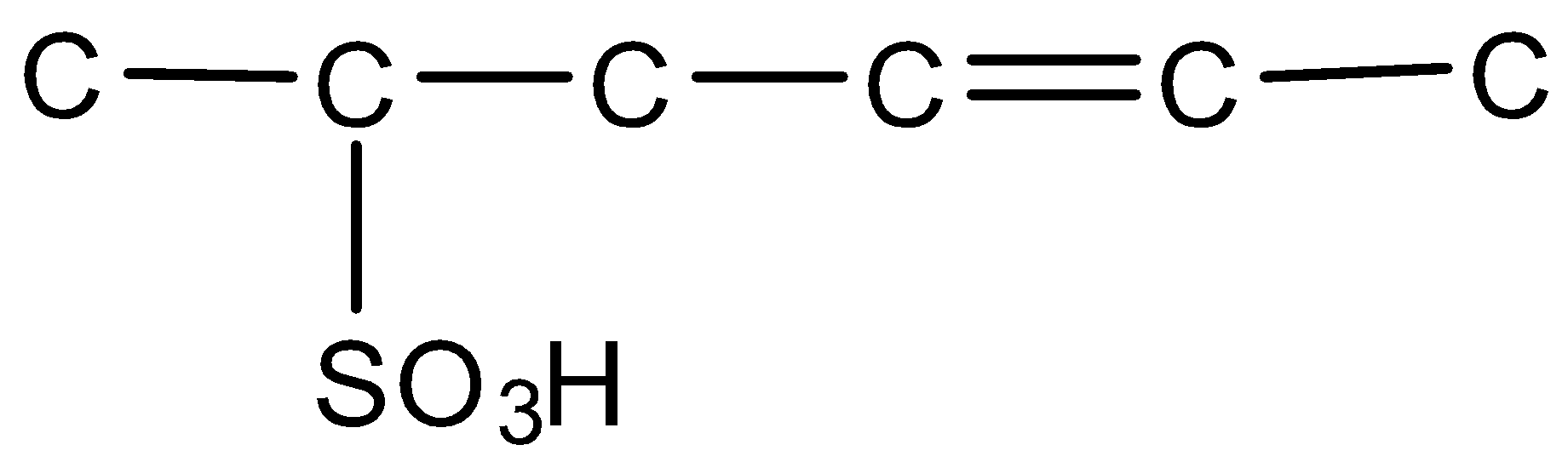
Answer
567.3k+ views
Hint: The answer to this question is based on the simple concept of organic chemistry that deals with the writing of structure of the hydrocarbons based on the valency of carbon atoms.
Complete Solution :
We have studied in our lower classes about the very basic concepts of organic chemistry that deal mainly with the hydrocarbons and also naming the hydrocarbons.
- Now, let us see how we can add the required number of hydrogen atoms so that the structure gets the actual form and the carbon gets the actual valency.
- The hydrocarbons are those compounds which have carbon atoms in the backbone and are composed of at least one hydrogen atom with it.
- We know the valency of hydrogen atom is one and that of carbon atom is four which is based on the electronic configuration of these two atoms that is according to their atomic numbers.
- Since a carbon atom has 4 electrons in its outermost orbit of the atom, the valence electrons of carbon is four and all the four electrons forms the bonding pair of electrons with the other atom.
- Now, since here in the question they have asked to write the structure based on carbon – hydrogen bond, we shall add the required number of hydrogen atoms to each of the carbon atoms which makes the carbon atom to satisfy the valence.
Thus, the structure of the given compound therefore can be written as:
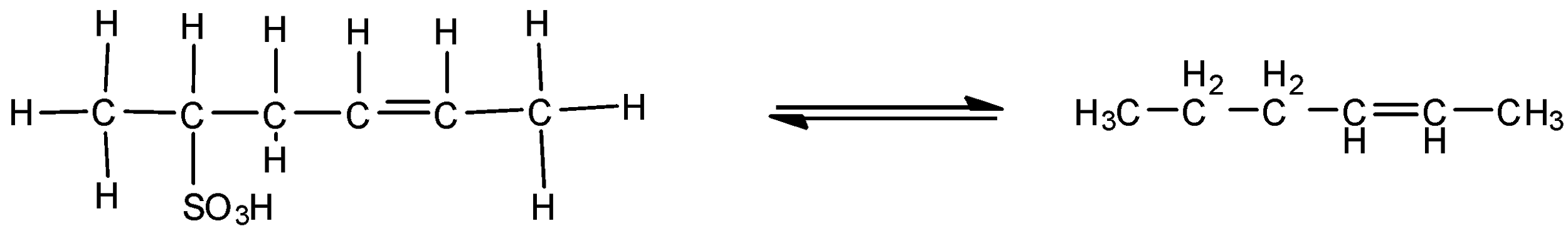
Note: Note that the writing of the structure is based on the total valence electrons that an atom can accommodate and thus filling of the required or given atom to fulfil the valency but if in case there is no mention of carbon hydrogen bonds even then by default the hydrogen atoms are to be filled.
Complete Solution :
We have studied in our lower classes about the very basic concepts of organic chemistry that deal mainly with the hydrocarbons and also naming the hydrocarbons.
- Now, let us see how we can add the required number of hydrogen atoms so that the structure gets the actual form and the carbon gets the actual valency.
- The hydrocarbons are those compounds which have carbon atoms in the backbone and are composed of at least one hydrogen atom with it.
- We know the valency of hydrogen atom is one and that of carbon atom is four which is based on the electronic configuration of these two atoms that is according to their atomic numbers.
- Since a carbon atom has 4 electrons in its outermost orbit of the atom, the valence electrons of carbon is four and all the four electrons forms the bonding pair of electrons with the other atom.
- Now, since here in the question they have asked to write the structure based on carbon – hydrogen bond, we shall add the required number of hydrogen atoms to each of the carbon atoms which makes the carbon atom to satisfy the valence.
Thus, the structure of the given compound therefore can be written as:

Note: Note that the writing of the structure is based on the total valence electrons that an atom can accommodate and thus filling of the required or given atom to fulfil the valency but if in case there is no mention of carbon hydrogen bonds even then by default the hydrogen atoms are to be filled.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




