
Draw the atomic structure of chlorine atom and chloride ion.
Answer
566.1k+ views
Hint:The atomic structure consists of electrons, protons and neutrons. The proton and neutron are placed in the nucleus and electrons are placed in different orbits around the nucleus. An ion is an electron rich or electron deficient species of the corresponding atom.
Complete step by step answer:
Chlorine is an atom in the periodic table with atomic number \[17\] and mass number \[35\]. The electronic configuration of chlorine is:
$Cl:1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
The valence shell of chlorine atom is \[3\]. It has a total of \[17\] electrons and \[17\] protons. The number of neutrons =\[35 - 17 = 18\]. The \[17\] protons and \[18\] neutrons are placed in the nucleus of chlorine. The \[17\] electrons are placed in different shells of chlorine atoms.
The shells around the nucleus of an atom are labelled as\[K\] , \[L\] , \[M\] , \[N\] and so on. The first shell \[K = 1\] has two electrons, the second shell \[K = 2\] has eight electrons and the third shell \[M = 3\] has seven electrons.
Thus the atomic structure of chlorine atoms is shown in figure \[1\] .
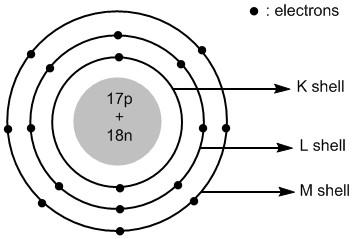
Figure \[1\] : Atomic structure of chlorine atom.
Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is \[18\] . The electronic configuration of chloride ion is:
$C{l^ - }:1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}$
In chloride ion the extra electron is added to the valence shell i.e. third shell or \[M\] shell. Thus the atomic structure of chloride ions is shown in figure \[2\] .
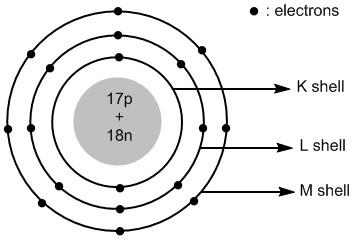
Figure \[2\] : Atomic structure of chloride ions.
Note:
Like the variance of the number of electrons in chlorine and chloride ions, the number of neutrons also varies. Chlorine exists in two isotopes with mass numbers \[35\] and\[37\] . Hence the number of neutrons in \[Cl - 35\] is \[18\] while in \[Cl - 37\] is \[20\].
Complete step by step answer:
Chlorine is an atom in the periodic table with atomic number \[17\] and mass number \[35\]. The electronic configuration of chlorine is:
$Cl:1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
The valence shell of chlorine atom is \[3\]. It has a total of \[17\] electrons and \[17\] protons. The number of neutrons =\[35 - 17 = 18\]. The \[17\] protons and \[18\] neutrons are placed in the nucleus of chlorine. The \[17\] electrons are placed in different shells of chlorine atoms.
The shells around the nucleus of an atom are labelled as\[K\] , \[L\] , \[M\] , \[N\] and so on. The first shell \[K = 1\] has two electrons, the second shell \[K = 2\] has eight electrons and the third shell \[M = 3\] has seven electrons.
Thus the atomic structure of chlorine atoms is shown in figure \[1\] .

Figure \[1\] : Atomic structure of chlorine atom.
Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is \[18\] . The electronic configuration of chloride ion is:
$C{l^ - }:1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}$
In chloride ion the extra electron is added to the valence shell i.e. third shell or \[M\] shell. Thus the atomic structure of chloride ions is shown in figure \[2\] .

Figure \[2\] : Atomic structure of chloride ions.
Note:
Like the variance of the number of electrons in chlorine and chloride ions, the number of neutrons also varies. Chlorine exists in two isotopes with mass numbers \[35\] and\[37\] . Hence the number of neutrons in \[Cl - 35\] is \[18\] while in \[Cl - 37\] is \[20\].
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




