
Draw lewis structures for the following molecules $HCOOH$ and $CO_2^{ - 2}$
Answer
573.6k+ views
Hint: Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.. They are also known as electron dot structure. It adds up two lines between the atoms that shows shared pairs.
Complete step by step answer:
tends to bond in such a way that each atom has $8$ electrons in its valence shell, giving it the same electronic configuration as inert gas
Now I am explaining steps for drawing lewis structure
Step $1$ :- firstly find the full number of valence electrons of the atoms .For anion , the whole charge is added and for cation, the full positive change is subtracted from the quantity of valence electrons .
Step $2$:- the smallest amount electronegative atom is placed at the centre, surrounded by highly electronegative atoms .
Step $3$ :-Draw a single bond among each atom, so complete octet rule of the encircling atoms followed by the central atom by keeping lone pair, covalent bond or triple bond.
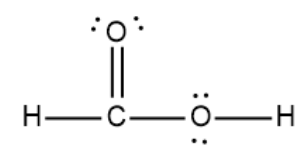
The above structure is lewis structure for $HCOOH$
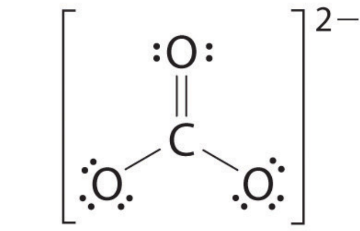
The above structure is lewis structure for $CO_2^{ - 2}$
Additional information:
The main difference between Lewis dot symbol and Lewis structure is that the Lewis dot symbol can represent the valence electrons present in the atom whereas a Lewis structure represents the structure of molecules with the help of symbols for chemical elements and dot symbols.
Note: The octet rule exists because the atoms of the many elements become more stable by attaining an element electron configuration. In this rule, atoms prefer to have eight electrons in the valence shell. The VSEPR theory describes main shapes of easy molecules: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral
Complete step by step answer:
tends to bond in such a way that each atom has $8$ electrons in its valence shell, giving it the same electronic configuration as inert gas
Now I am explaining steps for drawing lewis structure
Step $1$ :- firstly find the full number of valence electrons of the atoms .For anion , the whole charge is added and for cation, the full positive change is subtracted from the quantity of valence electrons .
Step $2$:- the smallest amount electronegative atom is placed at the centre, surrounded by highly electronegative atoms .
Step $3$ :-Draw a single bond among each atom, so complete octet rule of the encircling atoms followed by the central atom by keeping lone pair, covalent bond or triple bond.

The above structure is lewis structure for $HCOOH$

The above structure is lewis structure for $CO_2^{ - 2}$
Additional information:
The main difference between Lewis dot symbol and Lewis structure is that the Lewis dot symbol can represent the valence electrons present in the atom whereas a Lewis structure represents the structure of molecules with the help of symbols for chemical elements and dot symbols.
Note: The octet rule exists because the atoms of the many elements become more stable by attaining an element electron configuration. In this rule, atoms prefer to have eight electrons in the valence shell. The VSEPR theory describes main shapes of easy molecules: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




