
Draw labelled diagram to show angle of contact between
(i) Pure water and clean glass
(ii) Mercury and clean glass
Answer
514.2k+ views
Hint:The angle of contact is the angle between the tangent at the liquid surface and the solid surface. Angle of contact depends upon the nature of the liquid and the medium which exists above the free surface of liquid. Water is hydrophilic fluid and it makes an acute angle, whereas mercury is hydrophobic fluid and it makes an obtuse angle.
Complete answer:
Before designing the diagram, let us understand the angle of contact. The angle of contact is formed due to the surface tension of a fluid. In fluids, the forces on molecules due to the other molecules are balanced in all directions for the molecules inside the liquid. However for the surface of the liquid, these forces are not balanced and hence they tend to stretch the surface of fluid due to which it makes either a convex or concave meniscus.
The angle of contact can be defined as the angle made by the tangent at the liquid surface with the surface of solid inside the liquid. The angle of contact depends upon the nature of the liquid and the material of the surface in contact.Fluids are basically of two types – Hydrophilic and Hydrophobic.
Hydrophilic liquids are the liquids that are attracted to the surface of solid in contact and they wet the surface of solid. Hydrophilic liquids make an acute angle of contact with the surface. Hydrophilic fluids when filled in a capillary tube, rises in a capillary. Normal water is an example of hydrophilic fluid and thus it makes an acute angle with the surface.
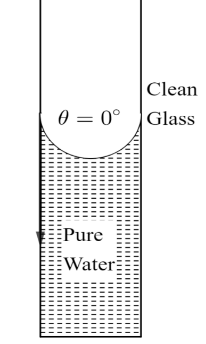
However, pure water does not have any kind of impurities in it. Hence, the angle of contact between the tangent at the liquid surface and the surface of clean glass will be $0^\circ$ as shown in figure.
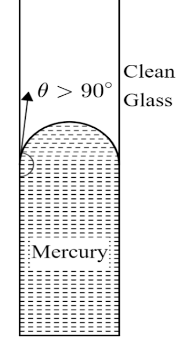
Hydrophobic liquids are the liquids that are repelled from the surface of solid in contact and they do not wet the surface of solid. Hydrophobic liquids make an obtuse angle of contact with the surface. Hydrophobic fluids when filled in a capillary tube, drops in a capillary.Mercury is an example of hydrophobic fluid and thus it makes an obtuse angle with the surface as shown in the figure below.
Note:Here, we have used an example of meniscus to show the angle of contact of pure water and mercury because due to the small area, the angle is visible clearly. Also we should note that here we are given pure water. For normal water, the angle of contact is acute but never $0^ {\circ}$ . The angle of contact is always measured within the liquid and is tangent to the surface of liquid.
Complete answer:
Before designing the diagram, let us understand the angle of contact. The angle of contact is formed due to the surface tension of a fluid. In fluids, the forces on molecules due to the other molecules are balanced in all directions for the molecules inside the liquid. However for the surface of the liquid, these forces are not balanced and hence they tend to stretch the surface of fluid due to which it makes either a convex or concave meniscus.
The angle of contact can be defined as the angle made by the tangent at the liquid surface with the surface of solid inside the liquid. The angle of contact depends upon the nature of the liquid and the material of the surface in contact.Fluids are basically of two types – Hydrophilic and Hydrophobic.
Hydrophilic liquids are the liquids that are attracted to the surface of solid in contact and they wet the surface of solid. Hydrophilic liquids make an acute angle of contact with the surface. Hydrophilic fluids when filled in a capillary tube, rises in a capillary. Normal water is an example of hydrophilic fluid and thus it makes an acute angle with the surface.
However, pure water does not have any kind of impurities in it. Hence, the angle of contact between the tangent at the liquid surface and the surface of clean glass will be $0^\circ$ as shown in figure.

Hydrophobic liquids are the liquids that are repelled from the surface of solid in contact and they do not wet the surface of solid. Hydrophobic liquids make an obtuse angle of contact with the surface. Hydrophobic fluids when filled in a capillary tube, drops in a capillary.Mercury is an example of hydrophobic fluid and thus it makes an obtuse angle with the surface as shown in the figure below.

Note:Here, we have used an example of meniscus to show the angle of contact of pure water and mercury because due to the small area, the angle is visible clearly. Also we should note that here we are given pure water. For normal water, the angle of contact is acute but never $0^ {\circ}$ . The angle of contact is always measured within the liquid and is tangent to the surface of liquid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




