
How do you draw electron orbital diagrams?
Answer
559.2k+ views
Hint: The atom contains orbitals in its structure and the electrons are going to revolve around the nucleus in fixed orbits called stationary orbits around the nucleus. Electrons have two spin clockwise and anti-clockwise.
Complete step by step answer:
- In the question it is asked how we can draw the electron orbital diagram.
- There are lots of ways to represent the electron orbital diagram.
- One of the ways to represent the electron orbital diagram is as follows.
- Here we are going to use lines to represent orbitals and arrows to represent electrons.
- Take an example of Helium, it contains 2 electrons in 1s orbital.
- The representation of the electron orbital diagram for helium is as follows.
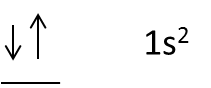
- The electrons in 1s orbital in opposite directions, one electron represented in clockwise direction and another electron represented in anti-clockwise direction.
- If we take a carbon element there are 6 electrons, the representation of electron orbital diagram for carbon atom is as follows.

- In carbon first two electrons are present in 1s orbital and next two electrons are going to enter 2s orbital.
- After filling the electrons in 2s orbital the remaining electrons are going to enter into 2p orbital which contains three subshells.
- This is how we can represent the electron orbital diagrams for different atoms.
Note: At the time of writing electron orbital diagrams we have to follow the electronic configuration of the particular atom. We should know the number of electrons in a particular atom to draw the electron orbital diagram.
Complete step by step answer:
- In the question it is asked how we can draw the electron orbital diagram.
- There are lots of ways to represent the electron orbital diagram.
- One of the ways to represent the electron orbital diagram is as follows.
- Here we are going to use lines to represent orbitals and arrows to represent electrons.
- Take an example of Helium, it contains 2 electrons in 1s orbital.
- The representation of the electron orbital diagram for helium is as follows.
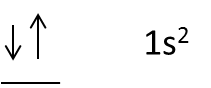
- The electrons in 1s orbital in opposite directions, one electron represented in clockwise direction and another electron represented in anti-clockwise direction.
- If we take a carbon element there are 6 electrons, the representation of electron orbital diagram for carbon atom is as follows.

- In carbon first two electrons are present in 1s orbital and next two electrons are going to enter 2s orbital.
- After filling the electrons in 2s orbital the remaining electrons are going to enter into 2p orbital which contains three subshells.
- This is how we can represent the electron orbital diagrams for different atoms.
Note: At the time of writing electron orbital diagrams we have to follow the electronic configuration of the particular atom. We should know the number of electrons in a particular atom to draw the electron orbital diagram.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




