
Draw electron dot structure of methanol.
Answer
544.2k+ views
Hint:To answer this question, you must recall the Lewis dot structures for various elements and how to draw them. Lewis structures of chemical species (elements or compounds) are the graphic representations of the distribution of electrons around the constituent atoms. The electron pairs are represented using dots.
Complete step-by-step answer:Methanol is an alcohol molecule with one carbon atom. The carbon atom is bonded to three hydrogen atoms and hydroxyl groups. We know that carbon has four valence electrons and requires four more to complete its octet. Hence it forms four covalent bonds. The oxygen- hydrogen bond in the hydroxyl group is also a covalent bond. The electron dot structure of methanol can be drawn as:
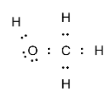
Note:Lewis structures are commonly used for predicting the number and the types of bonds that are formed by an atom. One must follow some steps when drawing the Lewis structure for species which are:
First, we need to calculate the total number of valence electrons present in the molecules by adding the separate individual valencies of the constituent atoms.
In case of any anions present, extra electrons are added as dots in the structure and in case of cations, electrons are subtracted.
The least electronegative atom in the molecule must be placed as the central atom with all other atoms placed around it with a single bond.
Lone pairs must be assigned first to the most electronegative atoms.
Now draw multiple bonds if needed in order to complete the octet of each atom in the molecule.
Complete step-by-step answer:Methanol is an alcohol molecule with one carbon atom. The carbon atom is bonded to three hydrogen atoms and hydroxyl groups. We know that carbon has four valence electrons and requires four more to complete its octet. Hence it forms four covalent bonds. The oxygen- hydrogen bond in the hydroxyl group is also a covalent bond. The electron dot structure of methanol can be drawn as:

Note:Lewis structures are commonly used for predicting the number and the types of bonds that are formed by an atom. One must follow some steps when drawing the Lewis structure for species which are:
First, we need to calculate the total number of valence electrons present in the molecules by adding the separate individual valencies of the constituent atoms.
In case of any anions present, extra electrons are added as dots in the structure and in case of cations, electrons are subtracted.
The least electronegative atom in the molecule must be placed as the central atom with all other atoms placed around it with a single bond.
Lone pairs must be assigned first to the most electronegative atoms.
Now draw multiple bonds if needed in order to complete the octet of each atom in the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




