
How do you draw and label Bohr models for O and P?
Answer
559.2k+ views
Hint: In order to draw a Bohr model for oxygen and Phosphorus, we must first know what a Bohr’s model is. To draw the Bohr model, we should know about the atomic number of Oxygen and Phosphorus.
Complete answer:
Let us see Bohr's model in detail. Bohr’s model consists of the nucleus and this nucleus is surrounded by the electrons that are revolving around it.
Let us see some of the postulates of Bohr’s model
- Atoms will consist of the positively charged nucleus in which the electrons will be revolving around it in a circular orbit or shell.
- Each orbit or shell will be having a definite energy.
- the energy level of the electrons is represented by an integer which is known as the quantum number. The orbits n = 1, 2, 3, 4 are designated as the K, L, M, N respectively.
- The electrons will gain energy and move from the lower to higher energy level. The electrons will lose energy and will move from the higher energy to the lower energy.
Let us now come to the given question.
The nucleus of the oxygen atom consists of the proton and neutrons. This nucleus is surrounded by 8 electrons. There will be two electrons in the K-shell and 6 electrons in the L-shell. The Bohr’s model of oxygen is given below:
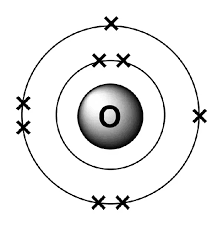
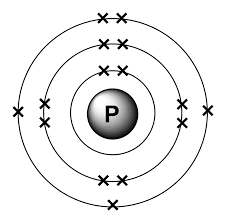
The nucleus of the phosphorus atom will also have neutrons and protons. This nucleus is surrounded by 15 electrons. There will be two electrons in the K-shell, 8 electrons in the L-shell and 5 electrons in the M-shell. The Bohr’s model for phosphorus is given below:
Note: We have to remember that the Bohr’s theory has certain shortcomings such as:
- Bohr’s model has failed to explain about the Zeeman effect.
- Bohr’s model has also failed to explain about the Stark effect.
- It did not explain about the spectra that is obtained from the larger atoms.
- Bohr’s model violates Heisenberg's uncertainty principle.
Complete answer:
Let us see Bohr's model in detail. Bohr’s model consists of the nucleus and this nucleus is surrounded by the electrons that are revolving around it.
Let us see some of the postulates of Bohr’s model
- Atoms will consist of the positively charged nucleus in which the electrons will be revolving around it in a circular orbit or shell.
- Each orbit or shell will be having a definite energy.
- the energy level of the electrons is represented by an integer which is known as the quantum number. The orbits n = 1, 2, 3, 4 are designated as the K, L, M, N respectively.
- The electrons will gain energy and move from the lower to higher energy level. The electrons will lose energy and will move from the higher energy to the lower energy.
Let us now come to the given question.
The nucleus of the oxygen atom consists of the proton and neutrons. This nucleus is surrounded by 8 electrons. There will be two electrons in the K-shell and 6 electrons in the L-shell. The Bohr’s model of oxygen is given below:

The nucleus of the phosphorus atom will also have neutrons and protons. This nucleus is surrounded by 15 electrons. There will be two electrons in the K-shell, 8 electrons in the L-shell and 5 electrons in the M-shell. The Bohr’s model for phosphorus is given below:

Note: We have to remember that the Bohr’s theory has certain shortcomings such as:
- Bohr’s model has failed to explain about the Zeeman effect.
- Bohr’s model has also failed to explain about the Stark effect.
- It did not explain about the spectra that is obtained from the larger atoms.
- Bohr’s model violates Heisenberg's uncertainty principle.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




