
How can I draw all possible stereoisomers for 2-chloro-3-hexanol?
Answer
548.4k+ views
Hint: Organic chemistry is the branch of chemistry in which carbon and its compounds are mainly studied. In Organic chemistry various compounds of carbon are found and formed as well. Carbon compounds are present in linear chain, branched chain and even in closed chain also. Carbon compounds have a unique property that they can arrange their atoms in different manner in three dimensional structure and due to which we obtain different structures of the same compounds.
Complete step by step answer:
Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other. Typically optical isomerism is shown by stereoisomers which rotate the plane of polarized light.
Chiral carbons are those which have no plane of symmetry and all the attached groups are different.
Achiral carbons are those where a plane of symmetry and all the groups attached to the central carbon atom are not different.
Molecules having chiral centre will shown optical isomerism first we will draw the bond-line structure of $2-$ chlorohexan$-3-01.$
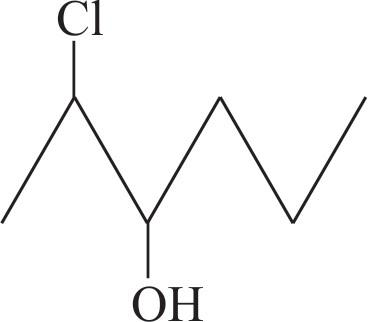
We can see that this compound contain two chiral centres : $\text{C}-2$ and $\text{C}-3$ $\to n=2$
So acc to $[{{2}^{n}}]$
$[{{2}^{n}}]=4$ stereoisomers
Here are the $4$ stereoisomer of $2$ chloro $-3-$ hexanol,
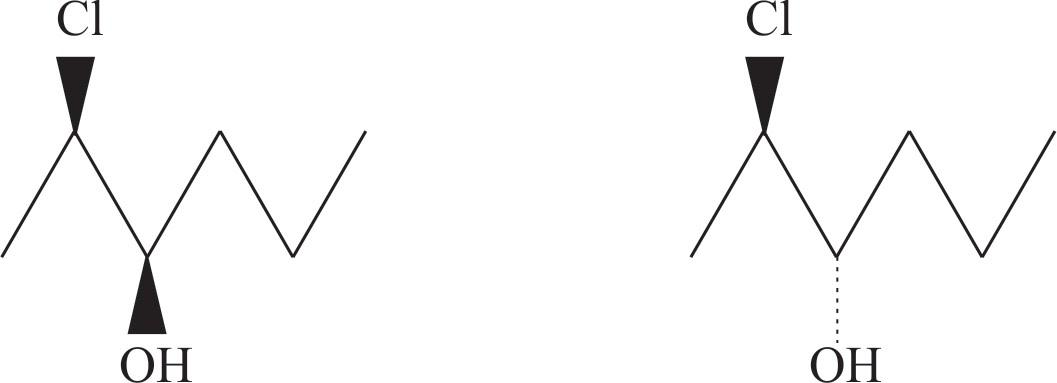
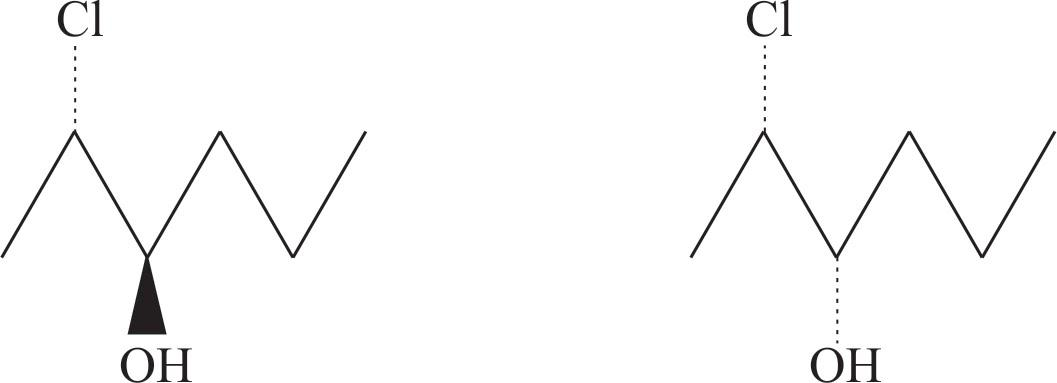
These are wedge-dash structures
Final Answer: The compound $2$ chloro$-3-$ hexanol has four possible stereoisomers.
Note: Isomerism is the phenomenon in which molecular formulas are the same but physical and chemical properties are different. Isomerism is further classified into two parts – Structural and Stereo-Isomerism.
In structural isomerism, species have similar molecular formula but the structure is different while in stereo isomerism, species have similar molecular formula but the arrangement of an atom in space is different. Due to their different arrangement they give rise to different molecules which have different either physical properties or chemical properties.
Complete step by step answer:
Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other. Typically optical isomerism is shown by stereoisomers which rotate the plane of polarized light.
Chiral carbons are those which have no plane of symmetry and all the attached groups are different.
Achiral carbons are those where a plane of symmetry and all the groups attached to the central carbon atom are not different.
Molecules having chiral centre will shown optical isomerism first we will draw the bond-line structure of $2-$ chlorohexan$-3-01.$

We can see that this compound contain two chiral centres : $\text{C}-2$ and $\text{C}-3$ $\to n=2$
So acc to $[{{2}^{n}}]$
$[{{2}^{n}}]=4$ stereoisomers
Here are the $4$ stereoisomer of $2$ chloro $-3-$ hexanol,
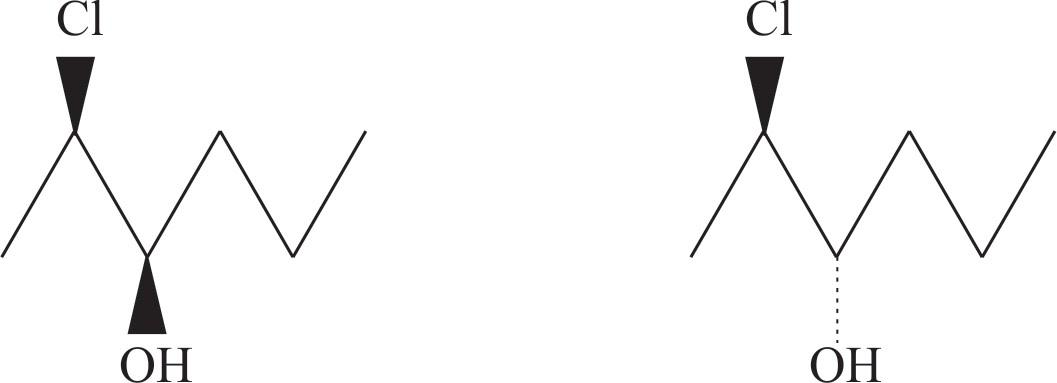
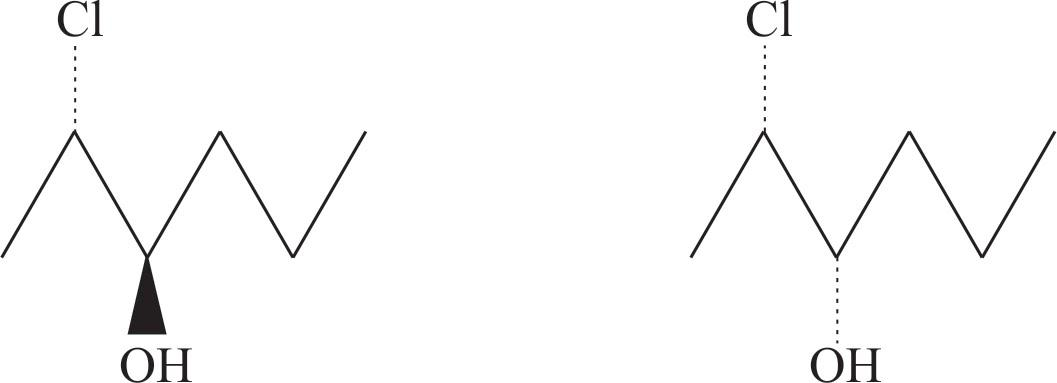
These are wedge-dash structures
Final Answer: The compound $2$ chloro$-3-$ hexanol has four possible stereoisomers.
Note: Isomerism is the phenomenon in which molecular formulas are the same but physical and chemical properties are different. Isomerism is further classified into two parts – Structural and Stereo-Isomerism.
In structural isomerism, species have similar molecular formula but the structure is different while in stereo isomerism, species have similar molecular formula but the arrangement of an atom in space is different. Due to their different arrangement they give rise to different molecules which have different either physical properties or chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




