
How can I draw a simple energy profile for an endothermic reaction on which 50 kj/mol is absorbed and which has an activation energy of 100 kj/mol?
Answer
547.5k+ views
Hint: In a chemical reaction if energy is absorbed by the reactants then the chemical reaction is called endothermic and if the energy is going to be released after the chemical reaction with the products then the reaction is called exothermic reaction.
Complete answer:
- In the question it is asked to draw the energy profile for an endothermic reaction by using the data given in the question.
- In the question it is given that 50 kj/mol energy is absorbed and the activation energy of the reactants to reach transition state is 100 kj/mol.
- Means the potential energy of the product is 50 kj/mol.
- We are supposed to take potential energy of ox y-axis and reaction pathway on x-axis while drawing the energy profile of any chemical reaction.
- Therefore the energy profile of the given endothermic reaction is as follows.
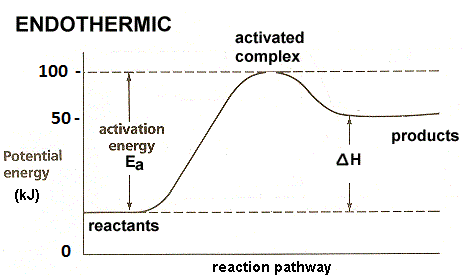
- From the above energy profile diagram we can say that the amount of energy required to activate the reactants is 100 kj/mol.
Note:
Every reactant in a chemical reaction needs some amount of energy to reach the transition state or to form an activated complex that minimum potential energy is called activation energy or transition energy. After the formation of the activated complex only we will get the products in a chemical reaction.
Complete answer:
- In the question it is asked to draw the energy profile for an endothermic reaction by using the data given in the question.
- In the question it is given that 50 kj/mol energy is absorbed and the activation energy of the reactants to reach transition state is 100 kj/mol.
- Means the potential energy of the product is 50 kj/mol.
- We are supposed to take potential energy of ox y-axis and reaction pathway on x-axis while drawing the energy profile of any chemical reaction.
- Therefore the energy profile of the given endothermic reaction is as follows.

- From the above energy profile diagram we can say that the amount of energy required to activate the reactants is 100 kj/mol.
Note:
Every reactant in a chemical reaction needs some amount of energy to reach the transition state or to form an activated complex that minimum potential energy is called activation energy or transition energy. After the formation of the activated complex only we will get the products in a chemical reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




