
Draw a neat diagram showing acid solution in water conducts electricity.
Answer
529.1k+ views
Hint: For the conduction of electricity we require ions which act as charge carriers in a solution. An acid can ionize to lose a proton and form hydronium ions (${H_3}{O^ + }$) which conducts electricity.
Complete Solution :
-First of all we should remember that for conduction of electricity we need the presence of charge carriers which can be ions or electrons. Also an electrolyte has the ability to dissociate to form its respective ions which can act as charge carriers and conduct electricity. Similarly an acid has the ability to release ${H^ + }$ ion and forms ions which can acid as charge carriers and thus conduct electricity.
-In simple form we can understand this as: Let us take an acid HCl which in water forms a solution:
$HX \to {H^ + } + C{l^ - }$
- These ${H^ + }$ ions cannot exist alone and so combine with ${H_2}O$ to form ${H_3}{O^ + }$ ions.
${H^ + } + {H_2}O \to {H_3}{O^ + }$
- The ${H_3}{O^ + }$ ions produced here at the end act as charge carriers and conduct electricity in the water solution of acid and water.
- This process is shown diagrammatically as below:
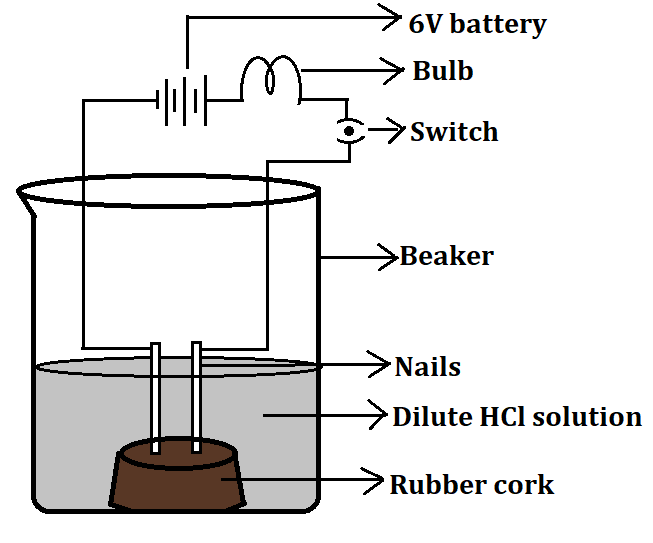
Note: All acid solutions including organic acids and mineral acids have the ability to conduct electricity. The mineral acids are stronger acids and thus dissociate completely to produce more number of ions, while the organic acids are weak acids and hence conduct electricity weakly. Hence mineral acids conduct electricity more strongly as compared to organic acids which are weak acids.
Complete Solution :
-First of all we should remember that for conduction of electricity we need the presence of charge carriers which can be ions or electrons. Also an electrolyte has the ability to dissociate to form its respective ions which can act as charge carriers and conduct electricity. Similarly an acid has the ability to release ${H^ + }$ ion and forms ions which can acid as charge carriers and thus conduct electricity.
-In simple form we can understand this as: Let us take an acid HCl which in water forms a solution:
$HX \to {H^ + } + C{l^ - }$
- These ${H^ + }$ ions cannot exist alone and so combine with ${H_2}O$ to form ${H_3}{O^ + }$ ions.
${H^ + } + {H_2}O \to {H_3}{O^ + }$
- The ${H_3}{O^ + }$ ions produced here at the end act as charge carriers and conduct electricity in the water solution of acid and water.
- This process is shown diagrammatically as below:

Note: All acid solutions including organic acids and mineral acids have the ability to conduct electricity. The mineral acids are stronger acids and thus dissociate completely to produce more number of ions, while the organic acids are weak acids and hence conduct electricity weakly. Hence mineral acids conduct electricity more strongly as compared to organic acids which are weak acids.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




