
Draw a labelled diagram to show the process of separation of miscible liquids.
Answer
503.7k+ views
Hint: If we are given two miscible liquids, it cannot be separated using primitive physical techniques like filtration, sedimentation, etc. Hence for two miscible liquids separation can be done based on their boiling points.
Complete answer:
The process of distillation is used to separate two miscible liquids. This process involves subsequent boiling and condensation of the mixture to separate out the various components. The process of distillation mainly runs on the principle of the difference in boiling point.
When the mixture of two components is heated on the Bunsen burner, the component with less boiling point goes into the gaseous state first. Simple distillation involves condensing the vapor immediately after it has been converted into the gaseous state. In the given procedure for distillation, the vapors pass through a condenser, which has water continuously flowing through the walls of it, to make sure that the temperature of the condenser is maintained at minimum, for the instant condensation of vapors. The condensed vapor of one component is then collected in a conical flask or beaker. In this way the various components of miscible liquid mixture are separated.
The labelled diagram can be given as
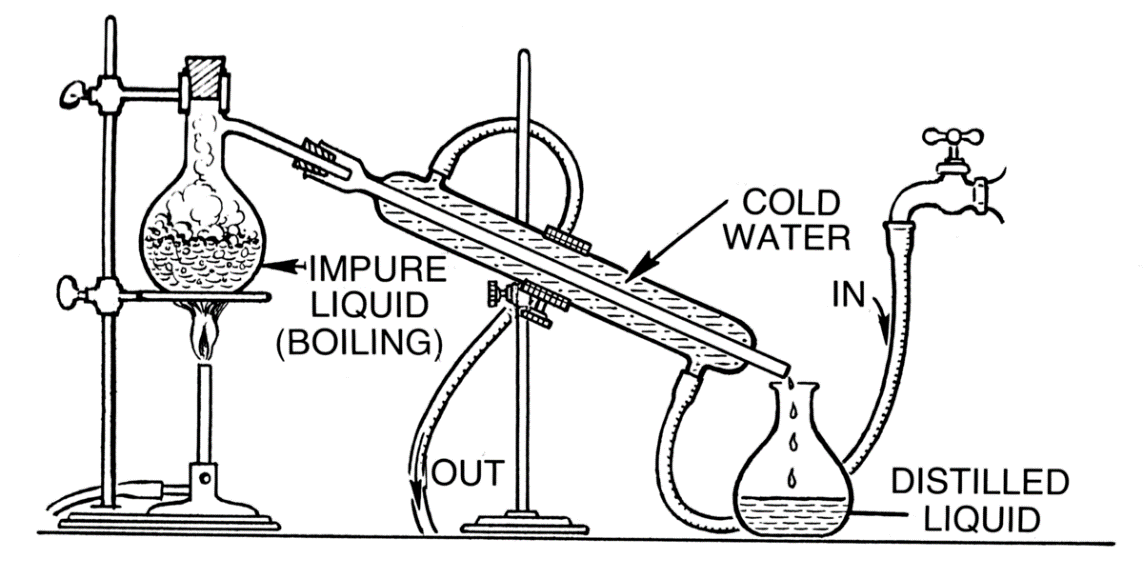
Impure liquid refers to the fixture of the miscible liquids having both the components and the distilled liquid is the pure liquid having only one component. The Round Bottom Flask having the impure mixture has a cork to avoid the vapors escaping out. Thermometer can also be inserted from the cork into the RBF, to note the temperature range for the vaporization of respective components.
Note:
The process of simple distillation has one limitation : it cannot be used to separate mixtures in which the components have a boiling point with very little difference. The two components should at least have a difference of $20 - {25^ \circ }C$ in their boiling points. For cases with lesser differences in boiling points, fractional distillation is used.
Complete answer:
The process of distillation is used to separate two miscible liquids. This process involves subsequent boiling and condensation of the mixture to separate out the various components. The process of distillation mainly runs on the principle of the difference in boiling point.
When the mixture of two components is heated on the Bunsen burner, the component with less boiling point goes into the gaseous state first. Simple distillation involves condensing the vapor immediately after it has been converted into the gaseous state. In the given procedure for distillation, the vapors pass through a condenser, which has water continuously flowing through the walls of it, to make sure that the temperature of the condenser is maintained at minimum, for the instant condensation of vapors. The condensed vapor of one component is then collected in a conical flask or beaker. In this way the various components of miscible liquid mixture are separated.
The labelled diagram can be given as

Impure liquid refers to the fixture of the miscible liquids having both the components and the distilled liquid is the pure liquid having only one component. The Round Bottom Flask having the impure mixture has a cork to avoid the vapors escaping out. Thermometer can also be inserted from the cork into the RBF, to note the temperature range for the vaporization of respective components.
Note:
The process of simple distillation has one limitation : it cannot be used to separate mixtures in which the components have a boiling point with very little difference. The two components should at least have a difference of $20 - {25^ \circ }C$ in their boiling points. For cases with lesser differences in boiling points, fractional distillation is used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




