
What Dow’s process in inorganic chemistry?
Answer
524.7k+ views
Hint: As we know that Dow’s process was a commercial method of preparation of phenol during the late ${{19}^{th}}$ and ${{20}^{th}}$century. So here we have to explain about this process which involves hydrolysis of chlorobenzene at high temperature and pressure conditions.
Complete answer:
Let us begin with the understanding of Dow’s process as follows:-
-Dow’s process: It is an early commercial method of preparation of phenol (in the late ${{19}^{th}}$ and early ${{20}^{th}}$ century) was through the hydrolysis of chlorobenzene with strong base like sodium hydroxide to produce a sodium phenoxide intermediate, which yields phenol upon acidification . The process is as follows:-
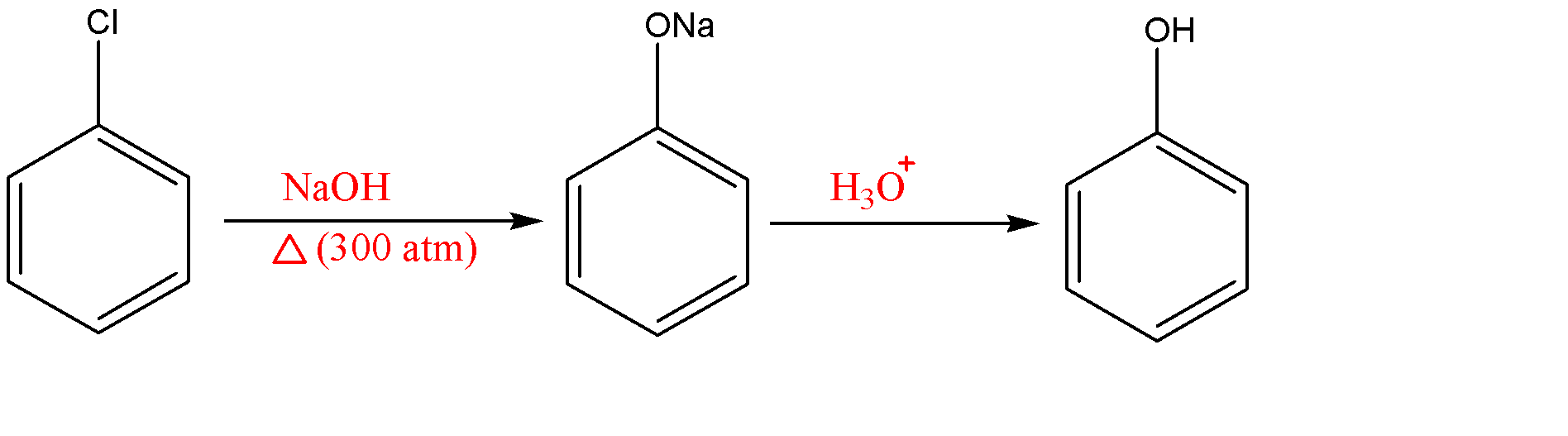
-We know that the starting reactant that is chlorobenzene can be easily produced from benzene through electrophilic aromatic substitution. This is then treated with aqueous sodium hydroxide at 350 $^{\circ }C$ and 300 atm or molten sodium hydroxide at 350 $^{\circ }C$ to convert it to sodium phenoxide as intermediate, which produces phenol upon final work up which is acidification.
-Also when 1-[$^{14}C$]-1-chlorobenzene is subjected to aqueous NaOH at 395$^{\circ }C$, ipso substitution product 1-[$^{14}C$]-phenol is formed with 54% yield whereas cine substitution product 2-[$^{14}C$]-phenol is formed with 43% yield which indicates that an elimination addition (benzyne) mechanism is predominant.
Note:
Remember that Dow’s process is also the electrolytic method of bromine extraction from brine where bromide containing brines are treated with sulfuric acid and bleaching powder so as to oxidize bromide to bromine, which remains dissolved in the water and further proceeds for filtration and distillation. This process is very costly as well as very complicated at the same time.
Complete answer:
Let us begin with the understanding of Dow’s process as follows:-
-Dow’s process: It is an early commercial method of preparation of phenol (in the late ${{19}^{th}}$ and early ${{20}^{th}}$ century) was through the hydrolysis of chlorobenzene with strong base like sodium hydroxide to produce a sodium phenoxide intermediate, which yields phenol upon acidification . The process is as follows:-
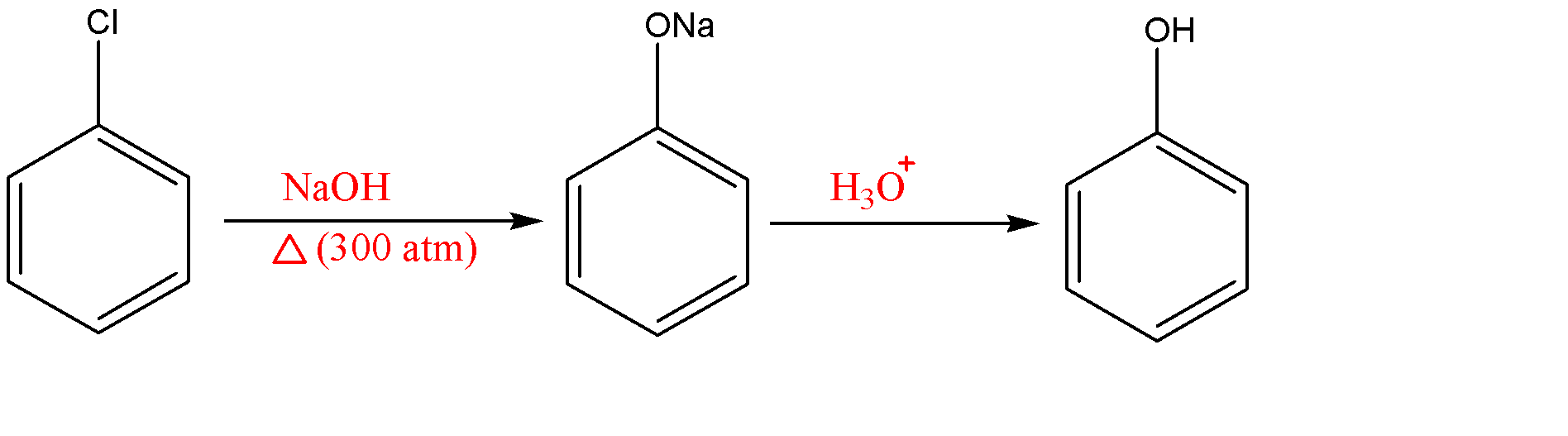
-We know that the starting reactant that is chlorobenzene can be easily produced from benzene through electrophilic aromatic substitution. This is then treated with aqueous sodium hydroxide at 350 $^{\circ }C$ and 300 atm or molten sodium hydroxide at 350 $^{\circ }C$ to convert it to sodium phenoxide as intermediate, which produces phenol upon final work up which is acidification.
-Also when 1-[$^{14}C$]-1-chlorobenzene is subjected to aqueous NaOH at 395$^{\circ }C$, ipso substitution product 1-[$^{14}C$]-phenol is formed with 54% yield whereas cine substitution product 2-[$^{14}C$]-phenol is formed with 43% yield which indicates that an elimination addition (benzyne) mechanism is predominant.
Note:
Remember that Dow’s process is also the electrolytic method of bromine extraction from brine where bromide containing brines are treated with sulfuric acid and bleaching powder so as to oxidize bromide to bromine, which remains dissolved in the water and further proceeds for filtration and distillation. This process is very costly as well as very complicated at the same time.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




