
How does kinetic energy relate to the states of matter?
Answer
547.5k+ views
Hint: Matter is something which is around us and occupies space. Matter exists in the three major forms Solid, liquid, gas. Kinetic energy is the energy by virtue of the motion of a particle. So, kinetic energy would be greater in that state of matter in which molecules will have free movement.
Complete step-by-step answer:We know, matter exists in solid liquid and gas. Let us see the organization of the molecules in each state and simultaneously we will also see the Kinetic energy relation with them.
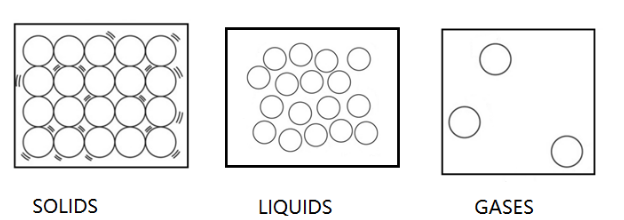
Solids: In solids we know that the molecules are compactly packed and thus solids have rigid structure. Compact packing means that the molecules are very close to each other. Due to the compact packing of molecules in solid, the molecules do not show movement. They just vibrate at their own place and thus have only the vibrational form of energy.
Thus, we can say that the kinetic energy of particles in the solid is least as they do not show movement.
Liquids: In liquids the molecules are little away from each other and thus they show movements. However, we should remember that the molecules are only at a few distances and not at a large distance from each other. Since the molecules in liquid show significant movement, they have a considerable amount of kinetic energy associated with them.
Thus, we can say that the kinetic energy of particles in the liquid is greater than that of solids.
Gas: In gases the molecules are very farther from each other and thus they show a wide range of movements. Gases can easily flow from one corner to another as their molecules show large amounts of movement. Since the molecules show large movement, kinetic energy associated with them is greatest.
Thus, they have the greatest kinetic energy.
The diagrammatic representation of the molecules of three states of matter are represented below.
Note:we should note that temperature can be defined in terms of the particle model to be a measure of the average kinetic energy of the particle. As we heat a substance we give more thermal energy to the particles which is converted to kinetic energy and hence they move apart.
Complete step-by-step answer:We know, matter exists in solid liquid and gas. Let us see the organization of the molecules in each state and simultaneously we will also see the Kinetic energy relation with them.
Solids: In solids we know that the molecules are compactly packed and thus solids have rigid structure. Compact packing means that the molecules are very close to each other. Due to the compact packing of molecules in solid, the molecules do not show movement. They just vibrate at their own place and thus have only the vibrational form of energy.
Thus, we can say that the kinetic energy of particles in the solid is least as they do not show movement.
Liquids: In liquids the molecules are little away from each other and thus they show movements. However, we should remember that the molecules are only at a few distances and not at a large distance from each other. Since the molecules in liquid show significant movement, they have a considerable amount of kinetic energy associated with them.
Thus, we can say that the kinetic energy of particles in the liquid is greater than that of solids.
Gas: In gases the molecules are very farther from each other and thus they show a wide range of movements. Gases can easily flow from one corner to another as their molecules show large amounts of movement. Since the molecules show large movement, kinetic energy associated with them is greatest.
Thus, they have the greatest kinetic energy.
The diagrammatic representation of the molecules of three states of matter are represented below.

Note:we should note that temperature can be defined in terms of the particle model to be a measure of the average kinetic energy of the particle. As we heat a substance we give more thermal energy to the particles which is converted to kinetic energy and hence they move apart.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




