
How does \[{H_2}{O_2}\] react within the acidic medium in either cold conditions?
Answer
566.7k+ views
Hint: To solve the question we can react hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] with potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\]. After the reaction, we can observe some changes under the suitable conditions of the reaction. Here we will react the reactants hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] and potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] in an acidic medium.
Complete answer:
First, we will write the properly balanced reaction of the given reactants under the given conditions. So here the reactants are hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] and potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\]. We need to react to the reactants in the presence of an acidic medium. We are considering acidic medium as sulphuric acid \[\left( {{H_2}S{O_4}} \right)\] in cold conditions. Now the reaction can be written as, \[4{H_2}{O_2} + {K_2}C{r_2}{O_7} + {H_2}S{O_4} \to 2Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
From the above-balanced chemical reaction, we can observe that when hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] and potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] reacts in the acidic medium the major product formed is chromic acid \[\left( {Cr{O_5}} \right)\] with potassium sulfate and water. The presence of chromic acid \[\left( {Cr{O_5}} \right)\] is the blue-colored formed acid.
Additional Details:
Other than this we can explore the structure of the major product chromic acid \[\left( {Cr{O_5}} \right)\] formed. The structure of chromic acid is represented as
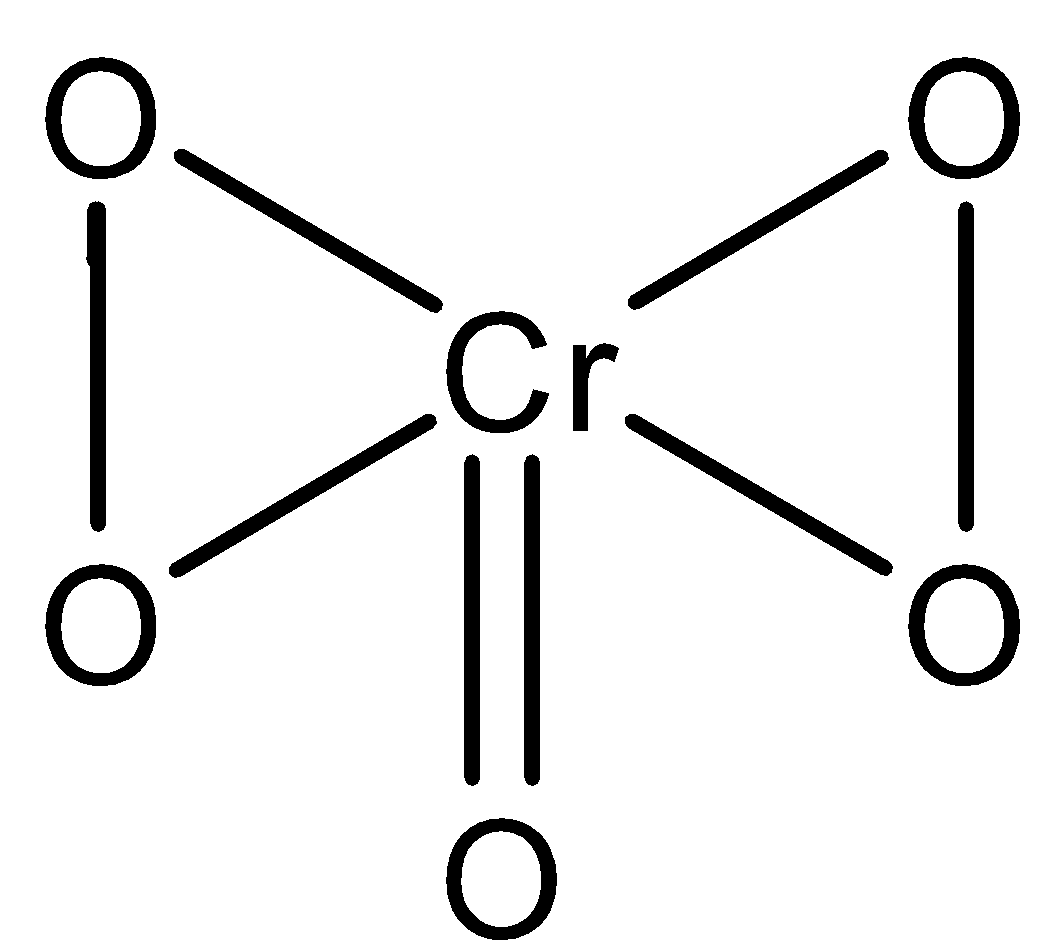 .
.
-By observing the structure we can note that four oxygen atoms are linked to the central atom by peroxide linkages. We can also calculate the oxidation state of chromic acid if we know the oxidation states of other oxygen atoms. The four oxygen are linked by peroxide linkage. Therefore, the oxidation state of the four oxygens will be \[ - 1\]. The other oxygen atom is in \[ - 2\] an oxidation state. Therefore, the oxidation state \[Cr\] is \[x + 4 \times \left( { - 1} \right) + \left( { - 2} \right) = 0,x = 6\].
Note:
Chromic acid is commonly known as chromium peroxide. The structure of chromic acid \[\left( {Cr{O_5}} \right)\] is like a butterfly. In cold conditions, chromic acid is stabilized by the ice-cold ether. There are \[4\] peroxy-linkages in chromic acid.
Complete answer:
First, we will write the properly balanced reaction of the given reactants under the given conditions. So here the reactants are hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] and potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\]. We need to react to the reactants in the presence of an acidic medium. We are considering acidic medium as sulphuric acid \[\left( {{H_2}S{O_4}} \right)\] in cold conditions. Now the reaction can be written as, \[4{H_2}{O_2} + {K_2}C{r_2}{O_7} + {H_2}S{O_4} \to 2Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
From the above-balanced chemical reaction, we can observe that when hydrogen peroxide \[\left( {{H_2}{O_2}} \right)\] and potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] reacts in the acidic medium the major product formed is chromic acid \[\left( {Cr{O_5}} \right)\] with potassium sulfate and water. The presence of chromic acid \[\left( {Cr{O_5}} \right)\] is the blue-colored formed acid.
Additional Details:
Other than this we can explore the structure of the major product chromic acid \[\left( {Cr{O_5}} \right)\] formed. The structure of chromic acid is represented as

-By observing the structure we can note that four oxygen atoms are linked to the central atom by peroxide linkages. We can also calculate the oxidation state of chromic acid if we know the oxidation states of other oxygen atoms. The four oxygen are linked by peroxide linkage. Therefore, the oxidation state of the four oxygens will be \[ - 1\]. The other oxygen atom is in \[ - 2\] an oxidation state. Therefore, the oxidation state \[Cr\] is \[x + 4 \times \left( { - 1} \right) + \left( { - 2} \right) = 0,x = 6\].
Note:
Chromic acid is commonly known as chromium peroxide. The structure of chromic acid \[\left( {Cr{O_5}} \right)\] is like a butterfly. In cold conditions, chromic acid is stabilized by the ice-cold ether. There are \[4\] peroxy-linkages in chromic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




