
Why does ethanol have a higher boiling point than ethane?
Answer
588.9k+ views
Hint: Think about the molecular structure of both the molecules and also the reactivity of the atoms that are involved in making the molecule. Consider the type of bonding these differences in electronegativity may contribute to.
Complete step by step solution:
Ethane has the molecular formula ${{C}_{2}}{{H}_{6}}$ and ethanol has the molecular formula of ${{C}_{2}}{{H}_{5}}OH$. Here, we can see that an extra oxygen atom is present in ethanol due to the presence of the hydroxyl group.
The higher boiling point of ethanol indicates that it has stronger or a larger number of bonds than ethane. Let us look at the structures of both the molecules before we determine why ethanol has a higher boiling point.
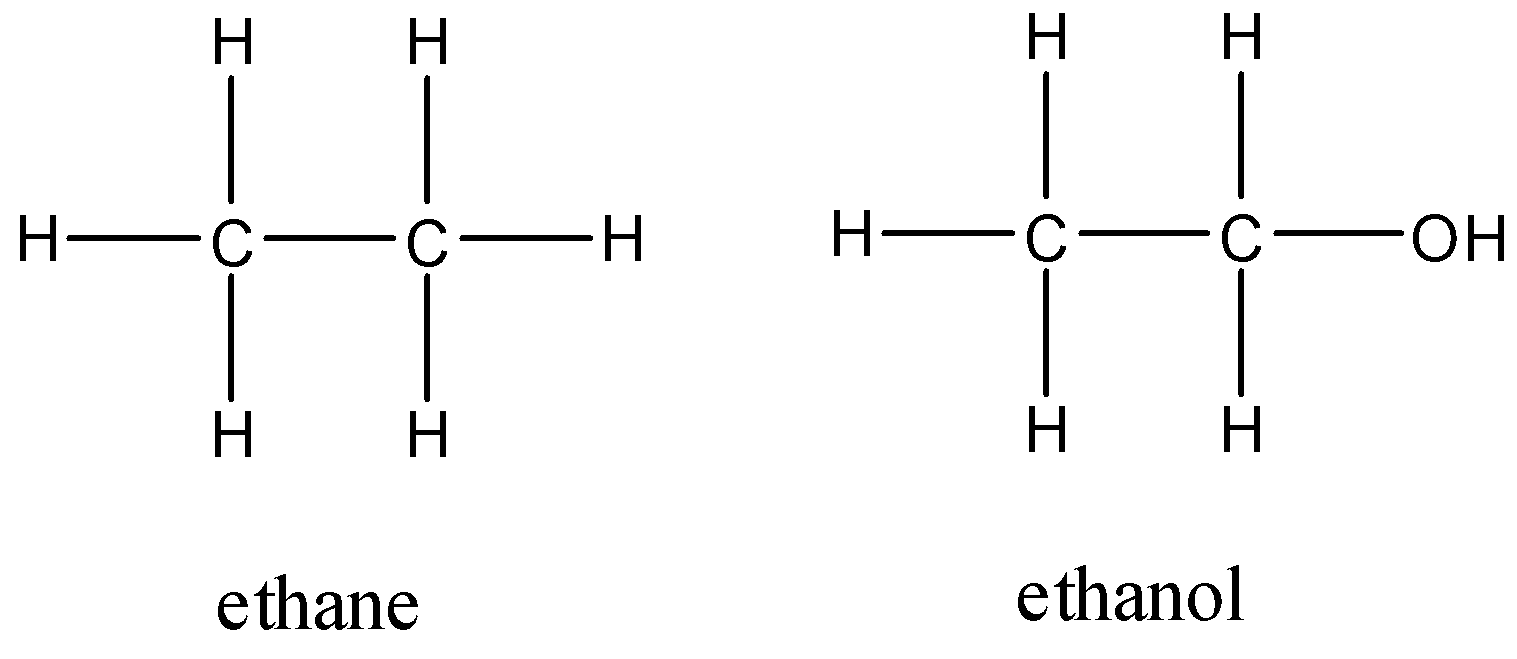
Despite the greater number of bonds present, this will not have an effect on the boiling point since the constituent molecule does not dissociate. The difference in electronegativity between carbon and oxygen will create a partial negative charge on oxygen.
This will cause the oxygen to form intermolecular bonds with the hydrogen atoms on the other molecules to form hydrogen bonds. The hydrogen atoms that are involved in the hydrogen bonds are the hydrogen atoms in the hydroxyl group and not the hydrogen atoms attached to the carbon atoms. The difference in electronegativity of carbon and hydrogen is very less, so partial charges will not develop on them. A visual representation of that is:
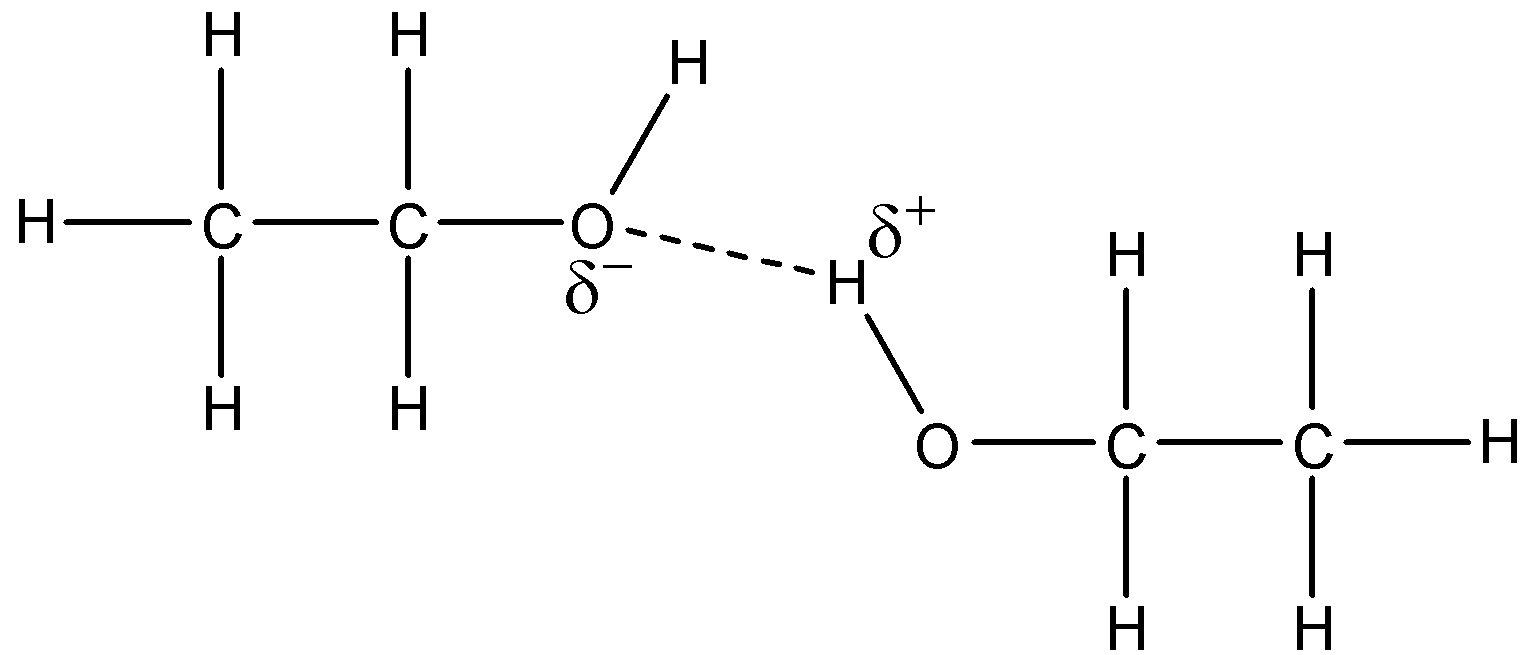
This hydrogen bonding is absent in the ethane molecules. Hence, its boiling point is lower.
Note: Remember that when a stronger or larger number of bonds are referred to in the context of boiling or melting points, they almost always refer to intermolecular bonding and not intra-molecular. Melting or boiling is a physical change and not a chemical one, so the molecule does not dissociate and the intramolecular bonds are not taken into consideration.
Complete step by step solution:
Ethane has the molecular formula ${{C}_{2}}{{H}_{6}}$ and ethanol has the molecular formula of ${{C}_{2}}{{H}_{5}}OH$. Here, we can see that an extra oxygen atom is present in ethanol due to the presence of the hydroxyl group.
The higher boiling point of ethanol indicates that it has stronger or a larger number of bonds than ethane. Let us look at the structures of both the molecules before we determine why ethanol has a higher boiling point.

Despite the greater number of bonds present, this will not have an effect on the boiling point since the constituent molecule does not dissociate. The difference in electronegativity between carbon and oxygen will create a partial negative charge on oxygen.
This will cause the oxygen to form intermolecular bonds with the hydrogen atoms on the other molecules to form hydrogen bonds. The hydrogen atoms that are involved in the hydrogen bonds are the hydrogen atoms in the hydroxyl group and not the hydrogen atoms attached to the carbon atoms. The difference in electronegativity of carbon and hydrogen is very less, so partial charges will not develop on them. A visual representation of that is:

This hydrogen bonding is absent in the ethane molecules. Hence, its boiling point is lower.
Note: Remember that when a stronger or larger number of bonds are referred to in the context of boiling or melting points, they almost always refer to intermolecular bonding and not intra-molecular. Melting or boiling is a physical change and not a chemical one, so the molecule does not dissociate and the intramolecular bonds are not taken into consideration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




