
How does benzene differ from hexane?
Answer
558.6k+ views
Hint:First we need to have an idea about hydrocarbons and its structures. It is evident that benzene and hexane are hydrocarbons in nature but they are different in structure and general formula. Therefore we should have a clear idea on its structure and general formula to substantiate the difference between both. Here we need to know benzene is an aromatic compound whereas hexane is an aliphatic compound.
Complete answer:
First we shall look into the case of benzene.
Let us consider a class of hydrocarbons where molecular formulas are the same as that of unsaturated hydrocarbons but the main thing to notice here is they do not undergo additional reactions unlike alkenes. Such compounds are placed in a distinct class known as aromatic hydrocarbons. It is also having unique structure and properties. One of such simplest compounds is benzene, having formula \[{C_6}{H_6}\] .
\[{C_6}{H_6}\] formula of benzene indicates its high degree of unsaturation . Benzene is having four degrees of unsaturation. Here another point is each degree of unsaturation implies \[C = C\] junction as in ethylene or ring junction. Benzene is an aromatic compound which is constrained in a ring structure. The ring structure of benzene comprises alternate single and double bonds. Benzene is having structure as below,
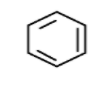
Next we have to look onto alkane. Alkane has a general formula \[{C_n}{H_{2n + 2}}\] which is fully saturated . In this , each two hydrogen less than this formula specifies one degree of saturation. Here the hexane comes under this alkane group.
Hexane is an aliphatic compound which is not constrained in a ring. Hexane contains eight more hydrogen atoms than benzene in which the former is having the general formula \[{C_6}{H_{14}}\] . It has only single bonds that link \[C\] atoms to group together as well as \[C\] atoms to attach with hydrogen atoms. Hexane is having structure as below,
\[C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2} - C{H_3}\]
Note:We know benzene is an aromatic compound which is unsaturated. Due to this consequence of unsaturation, benzene can undergo aromatic electrophilic substitution. Hexane is an organic compound containing five isomeric aliphatic hydrocarbons which are colourless in nature and are volatile liquids. Also , an important point is that an unsaturated compound is also a saturated compound , which contains double or triple bonds but not having single bonds.
Complete answer:
First we shall look into the case of benzene.
Let us consider a class of hydrocarbons where molecular formulas are the same as that of unsaturated hydrocarbons but the main thing to notice here is they do not undergo additional reactions unlike alkenes. Such compounds are placed in a distinct class known as aromatic hydrocarbons. It is also having unique structure and properties. One of such simplest compounds is benzene, having formula \[{C_6}{H_6}\] .
\[{C_6}{H_6}\] formula of benzene indicates its high degree of unsaturation . Benzene is having four degrees of unsaturation. Here another point is each degree of unsaturation implies \[C = C\] junction as in ethylene or ring junction. Benzene is an aromatic compound which is constrained in a ring structure. The ring structure of benzene comprises alternate single and double bonds. Benzene is having structure as below,

Next we have to look onto alkane. Alkane has a general formula \[{C_n}{H_{2n + 2}}\] which is fully saturated . In this , each two hydrogen less than this formula specifies one degree of saturation. Here the hexane comes under this alkane group.
Hexane is an aliphatic compound which is not constrained in a ring. Hexane contains eight more hydrogen atoms than benzene in which the former is having the general formula \[{C_6}{H_{14}}\] . It has only single bonds that link \[C\] atoms to group together as well as \[C\] atoms to attach with hydrogen atoms. Hexane is having structure as below,
\[C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2} - C{H_3}\]
Note:We know benzene is an aromatic compound which is unsaturated. Due to this consequence of unsaturation, benzene can undergo aromatic electrophilic substitution. Hexane is an organic compound containing five isomeric aliphatic hydrocarbons which are colourless in nature and are volatile liquids. Also , an important point is that an unsaturated compound is also a saturated compound , which contains double or triple bonds but not having single bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




