
What does a soda-acid type fire extinguisher contain? How does it work? Explain the working of a soda-acid fire extinguisher with the help of a labelled diagram.
Answer
551.4k+ views
Hint: A soda acid fire extinguisher contains an acid and a hydrogen carbonate, which react together to form carbon dioxide gas and this gas is responsible for stopping the fire.
Complete answer:
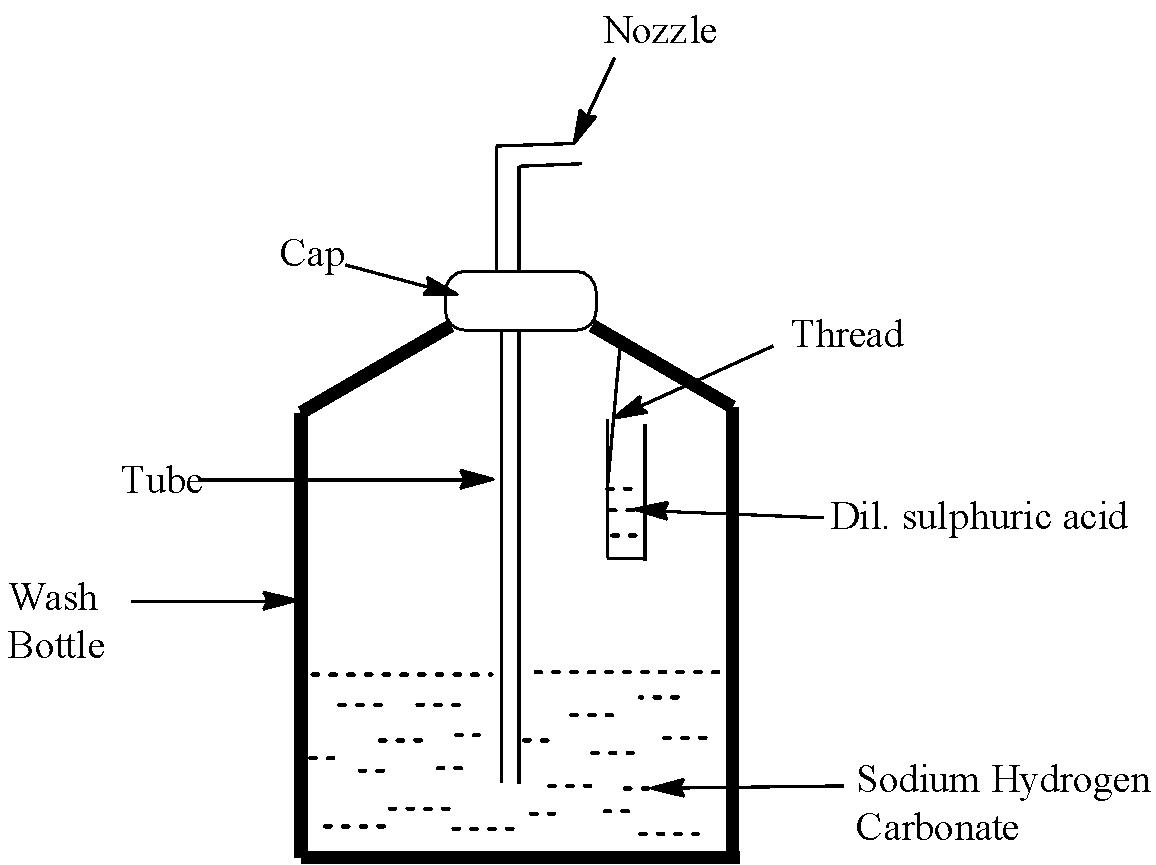
The soda acid fire extinguisher works on the principle of the products generated with the reaction of two compounds, namely sulphuric acid and sodium hydrogen carbonate. In a typical soda acid fire extinguisher, both of these compounds are present, but they are kept separately, in different chambers. Now, we know that carbon dioxide gas is a very good greenhouse gas and it can absorb a very good amount of heat. Carbon dioxide cuts off the layer between the fire and the oxygen of the atmosphere, thus, forming a layer, and this helps the fire to stop. The reaction between sodium hydrogen carbonate and sulphuric acid produces carbon dioxide gas.
Since the sulphuric acid and the sodium hydrogen carbonate are kept in two separate containers, they cannot react together. However, there is a knob, which when turned or pressed, mixes the two compounds and carbon dioxide is formed. This gas is then sprayed on the fire and it forms a blanket around the burning substance, and in sometime the fire gets extinguished. Let us see the reaction that takes place when a soda acid fire extinguisher comes in action:
\[{{H}_{2}}S{{O}_{4}}+NaHC{{O}_{3}}\to N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O+C{{O}_{2}}\]
Now, let us see the diagram of soda acid fire extinguisher:
Note:
Carbon dioxide comes out pressurised, so during the action of the extinguisher, the surroundings may feel cold. Moreover, there is a formation of a white powder all over the p
Complete answer:
The soda acid fire extinguisher works on the principle of the products generated with the reaction of two compounds, namely sulphuric acid and sodium hydrogen carbonate. In a typical soda acid fire extinguisher, both of these compounds are present, but they are kept separately, in different chambers. Now, we know that carbon dioxide gas is a very good greenhouse gas and it can absorb a very good amount of heat. Carbon dioxide cuts off the layer between the fire and the oxygen of the atmosphere, thus, forming a layer, and this helps the fire to stop. The reaction between sodium hydrogen carbonate and sulphuric acid produces carbon dioxide gas.
Since the sulphuric acid and the sodium hydrogen carbonate are kept in two separate containers, they cannot react together. However, there is a knob, which when turned or pressed, mixes the two compounds and carbon dioxide is formed. This gas is then sprayed on the fire and it forms a blanket around the burning substance, and in sometime the fire gets extinguished. Let us see the reaction that takes place when a soda acid fire extinguisher comes in action:
\[{{H}_{2}}S{{O}_{4}}+NaHC{{O}_{3}}\to N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O+C{{O}_{2}}\]
Now, let us see the diagram of soda acid fire extinguisher:

Note:
Carbon dioxide comes out pressurised, so during the action of the extinguisher, the surroundings may feel cold. Moreover, there is a formation of a white powder all over the p
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




