
How does a battery work? Explain
Answer
504.3k+ views
Hint: Battery is a device consisting of two or more galvanic cells connected in series or parallel. Batteries are a portable source of energy. Batteries can store chemical energy in the form of active materials and on demand convert into electrical energy through electrochemical redox reaction. Batteries are used in calculators, digital watches, hearing aids, laptops, car engines, space applications etc.
Complete answer:
LITHIUM-ION BATTERY
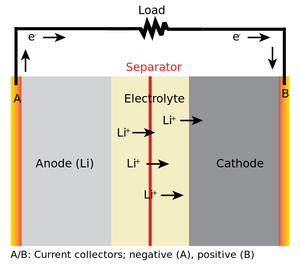
Cell representation:
${\text{Li|Li + , C|LiP}}{{\text{F}}_6}{\text{ in ethylene carbonate|LiCo}}{{\text{O}}_2}$
Working of LITHIUM-ION BATTERY:
Lithium-ion secondary batteries depend on an "intercalation" mechanism. During the charge and discharge processes, lithium ions are inserted or extracted from interstitial space between atomic layers within the active material, without changing the crystal structure. During discharging, Li atoms present in the graphite layer are oxidized to lithium ions and migrate through the organic electrolyte towards the cathode. Electrons flow through an external circuit. At cathode lithium ions are reduced to Li atoms and are inserted into the layered structure of metal oxide.
During charging of the battery, lithium atoms present in the layered structure of metal oxide are oxidized, liberating electrons and Li ions. Electrons flow through an external circuit and lithium ions flow through the organic electrolyte towards graphite carbon electrodes. At graphite electrodes, Li ions are reduced to Li atoms and are inserted into the layered structure of graphite. Thus, the lithium ions pass back and forth between the electrodes during charging and discharging. Because of this reason, the lithium-ion batteries are called ‘Rocking chair’, ‘Swing’ cells.
Electrode reaction:
Anodic reaction:
${\text{L}}{{\text{i}}_n}{\text{C6}}\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{6C + nL}}{{\text{i}}^ + } + {\text{n}}{{\text{e}}^ - }$
Cathodic reaction:
${\text{L}}{{\text{i}}_{1 - n}}{\text{Co}}{{\text{O}}_2} + {\text{nL}}{{\text{i}}^ + } + {\text{n}}{{\text{e}}^ - }\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{LiCo}}{{\text{O}}_2}$
Net cell reaction:
${\text{L}}{{\text{i}}_{1 - n}}{\text{Co}}{{\text{O}}_2} + {\text{L}}{{\text{i}}_n}{\text{C}}\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{LiCo}}{{\text{O}}_2} + {\text{C}}$
Additional information:
Lithium is a light metal with low electrode potential and good conductivity. It is therefore a good material for batteries and can be expected to have high potential and high energy density. The batteries where lithium is used as an anode are known as lithium batteries. These batteries have the following characteristics:
1. These batteries are light in weight and compact.
2. It has very low electrode potential (-3.04).
3. Good conductivity
4. It has a high charge density due to the low atomic mass of lithium.
5. It produces voltage up to 4 volts depending upon the cathode material
6. It can be operated over a wide range of temperatures i.e., from 70°C to -40°C.
7. Superior shelf life.
Note:
Battery:
The purpose of the battery and cells are the same, but a battery contains a number of cells in series or parallel to develop or produce a voltage of desired level of voltage.
Cell:
A cell is a form of an energy in which it generates only DC voltage and current of small magnitude.
Complete answer:
LITHIUM-ION BATTERY

Cell representation:
${\text{Li|Li + , C|LiP}}{{\text{F}}_6}{\text{ in ethylene carbonate|LiCo}}{{\text{O}}_2}$
Working of LITHIUM-ION BATTERY:
Lithium-ion secondary batteries depend on an "intercalation" mechanism. During the charge and discharge processes, lithium ions are inserted or extracted from interstitial space between atomic layers within the active material, without changing the crystal structure. During discharging, Li atoms present in the graphite layer are oxidized to lithium ions and migrate through the organic electrolyte towards the cathode. Electrons flow through an external circuit. At cathode lithium ions are reduced to Li atoms and are inserted into the layered structure of metal oxide.
During charging of the battery, lithium atoms present in the layered structure of metal oxide are oxidized, liberating electrons and Li ions. Electrons flow through an external circuit and lithium ions flow through the organic electrolyte towards graphite carbon electrodes. At graphite electrodes, Li ions are reduced to Li atoms and are inserted into the layered structure of graphite. Thus, the lithium ions pass back and forth between the electrodes during charging and discharging. Because of this reason, the lithium-ion batteries are called ‘Rocking chair’, ‘Swing’ cells.
Electrode reaction:
Anodic reaction:
${\text{L}}{{\text{i}}_n}{\text{C6}}\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{6C + nL}}{{\text{i}}^ + } + {\text{n}}{{\text{e}}^ - }$
Cathodic reaction:
${\text{L}}{{\text{i}}_{1 - n}}{\text{Co}}{{\text{O}}_2} + {\text{nL}}{{\text{i}}^ + } + {\text{n}}{{\text{e}}^ - }\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{LiCo}}{{\text{O}}_2}$
Net cell reaction:
${\text{L}}{{\text{i}}_{1 - n}}{\text{Co}}{{\text{O}}_2} + {\text{L}}{{\text{i}}_n}{\text{C}}\underset{{{\text{charging}}}}{\overset{{{\text{Discharging}}}}{\longleftrightarrow}}{\text{LiCo}}{{\text{O}}_2} + {\text{C}}$
Additional information:
Lithium is a light metal with low electrode potential and good conductivity. It is therefore a good material for batteries and can be expected to have high potential and high energy density. The batteries where lithium is used as an anode are known as lithium batteries. These batteries have the following characteristics:
1. These batteries are light in weight and compact.
2. It has very low electrode potential (-3.04).
3. Good conductivity
4. It has a high charge density due to the low atomic mass of lithium.
5. It produces voltage up to 4 volts depending upon the cathode material
6. It can be operated over a wide range of temperatures i.e., from 70°C to -40°C.
7. Superior shelf life.
Note:
Battery:
The purpose of the battery and cells are the same, but a battery contains a number of cells in series or parallel to develop or produce a voltage of desired level of voltage.
Cell:
A cell is a form of an energy in which it generates only DC voltage and current of small magnitude.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




