
Distinguish between diamond and graphite.
Answer
594.9k+ views
Hint: Mention their physical as well as chemical properties. Describe their structures.
Complete answer:
It is very common knowledge that both diamond and graphite are allotropes of carbon. Allotropes are compounds which are chemically the same but significantly vary in their physical properties. Before differentiating them, let us look at carbon first. It belongs to the second period and fourteenth group of the atomic table. It is therefore a metalloid which means its electronegativity and electropositivity are well balanced. As it is the first member of its group, carbon is the smallest among them and does not have d-orbitals. All of these properties make it an element which has the highest self-linking property among all the other elements in the periodic table. It is also able to form strong bonds with metals, nonmetals and metalloids. Diamond and graphite are large molecules of carbon which do not have a specific formula. Their size completely depends on the extent of their physical structures.
The table below summarizes the differences between diamond and graphite.
Note: Although both diamond and graphite have many significant physical differences, they are same in many chemical properties which more involve the fact that they are composed of the same element than their macrostructure and other conditions that gave birth to their physical differences.
Both of them are insoluble in organic solvents and also in polar solvents such as water. This is due to the fact that the covalent bonds are stronger and the energy released when they bond with water is far inferior to that.
Complete answer:
It is very common knowledge that both diamond and graphite are allotropes of carbon. Allotropes are compounds which are chemically the same but significantly vary in their physical properties. Before differentiating them, let us look at carbon first. It belongs to the second period and fourteenth group of the atomic table. It is therefore a metalloid which means its electronegativity and electropositivity are well balanced. As it is the first member of its group, carbon is the smallest among them and does not have d-orbitals. All of these properties make it an element which has the highest self-linking property among all the other elements in the periodic table. It is also able to form strong bonds with metals, nonmetals and metalloids. Diamond and graphite are large molecules of carbon which do not have a specific formula. Their size completely depends on the extent of their physical structures.
The table below summarizes the differences between diamond and graphite.
| DIAMOND | GRAPHITE |
1. The hybridisation of carbon here is $s{{p}^{3}}$. Therefore it has a tetrahedral structure.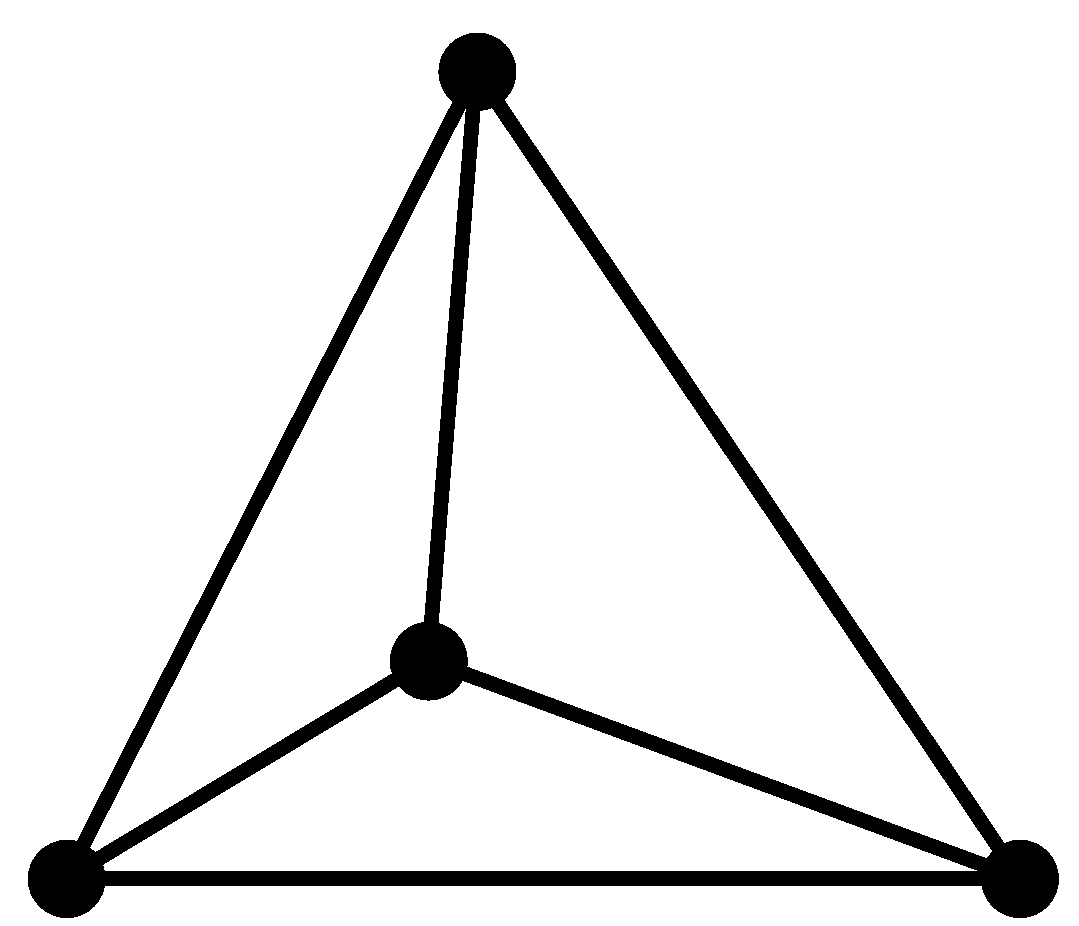
| 1. The hybridisation of carbon is$s{{p}^{2}}$. The structure is trigonal planar.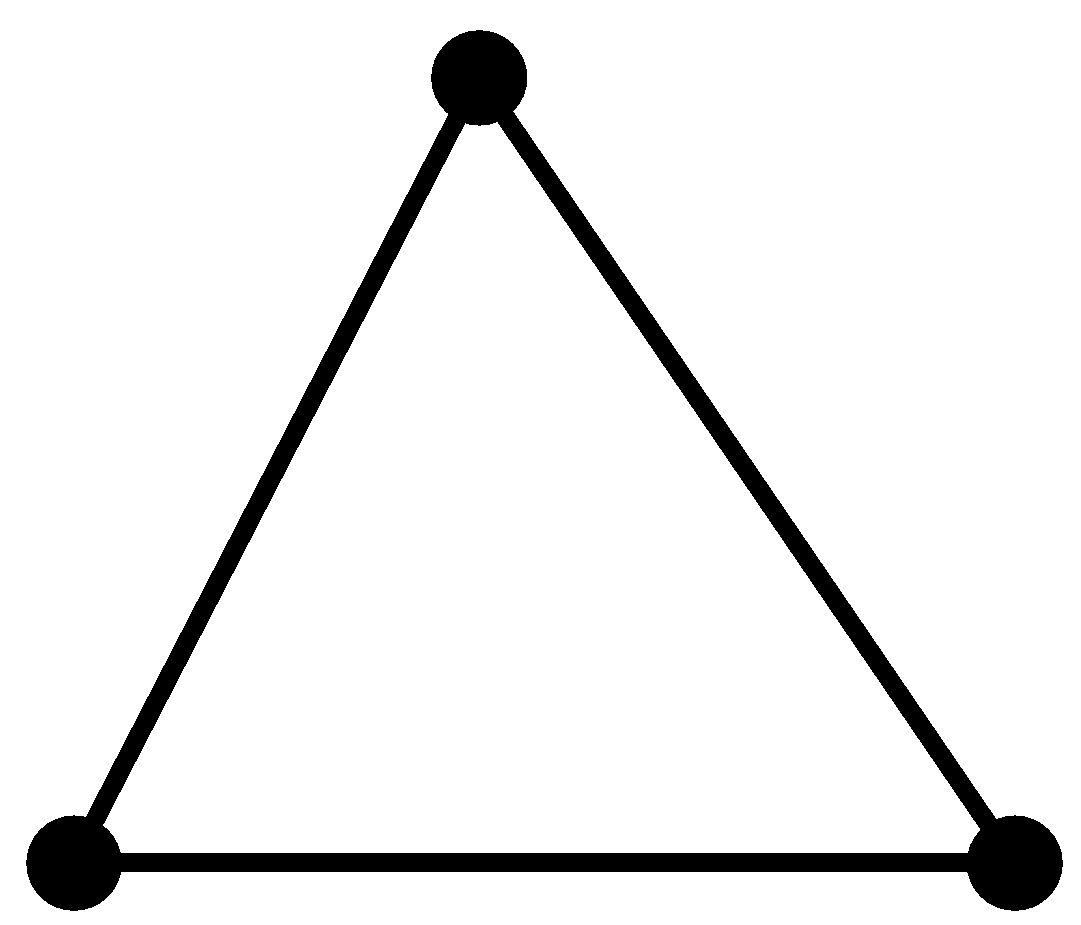
|
Note: Although both diamond and graphite have many significant physical differences, they are same in many chemical properties which more involve the fact that they are composed of the same element than their macrostructure and other conditions that gave birth to their physical differences.
Both of them are insoluble in organic solvents and also in polar solvents such as water. This is due to the fact that the covalent bonds are stronger and the energy released when they bond with water is far inferior to that.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




