
How will you distinguish among the following pairs?
n – Butyl, sec. butyl and tert. Butyl alcohols
Answer
512.7k+ views
Hint: Alcohols are classified as primary, secondary and tertiary. The alcohol group attached with only 1 carbon is termed as primary; alcohol attached to 2 other carbons is secondary and with 3 carbon atoms is tertiary alcohol. Alcohols are named as n, sec, and tert with their arrangement of the alcohol group. The Lucas test will distinguish among these.
Complete answer:
We have been given a pair of alcohols as n – Butyl, sec. butyl and tert. Butyl alcohols. In n – butyl alcohol, the OH group is attached with one carbon atom, so it is primary$1{}^\circ $ alcohol. In sec – butyl alcohol, the OH group is attached with two carbon atoms, so it is secondary $2{}^\circ $alcohol. In tert – butyl alcohol, the OH group is attached with three carbon atoms, so it is tertiary $3{}^\circ $alcohol.
These $1{}^\circ ,2{}^\circ ,3{}^\circ $alcohols are distinguished by Lucas reagent test. Lucas reagent is anhydrous zinc chloride and hydrochloric acid in the ratio 1:1 $ZnC{{l}_{2}}+HCl$. By reacting with Lucas reagent, alcohols form halides (alkyl chlorides) which have variable solubility. So, on reaction,
n – butyl alcohol is a primary alcohol, so it will have no reaction with Lucas reagent as:
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow{ZnC{{l}_{2}}+HCl}no\,reaction$
Sec – butyl alcohol, will form a turbid solution after 5 minutes due to the formation of secondary halide as:
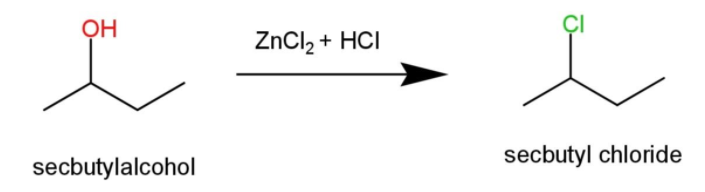
Tert – butyl alcohol will show immediate turbidity in the reaction as it is a tertiary halide as:
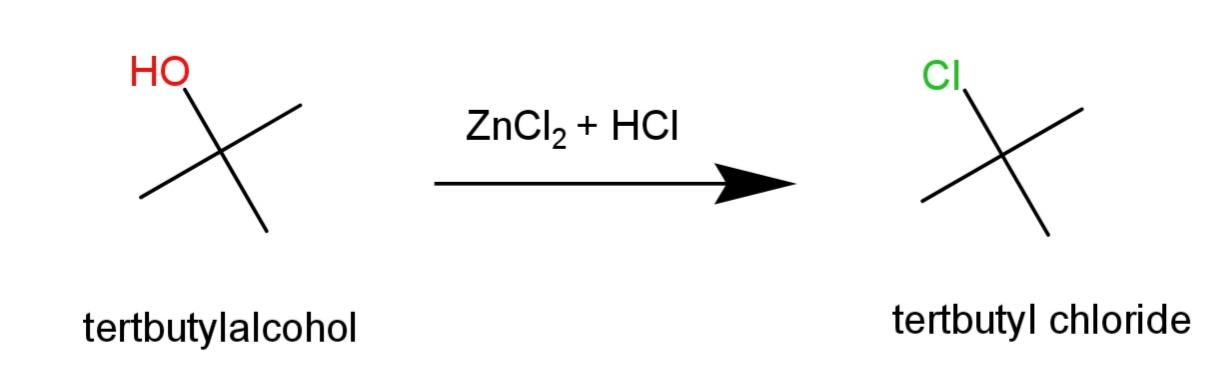
Hence, n – Butyl, sec – butyl and tert – Butyl alcohols can be distinguished by the turbidity formed on reaction with Lucas reagent that is $ZnC{{l}_{2}}+HCl$.
Note:
The decreasing order of the reaction of alcohols towards Lucas reagent is$3{}^\circ >2{}^\circ >1{}^\circ $ as tertiary alcohols will give immediate turbidity with Lucas reagent. This test is based on the fact that alcohols are soluble in Lucas reagent, but the halides are not soluble, so they will form a turbid solution with cloudiness.
Complete answer:
We have been given a pair of alcohols as n – Butyl, sec. butyl and tert. Butyl alcohols. In n – butyl alcohol, the OH group is attached with one carbon atom, so it is primary$1{}^\circ $ alcohol. In sec – butyl alcohol, the OH group is attached with two carbon atoms, so it is secondary $2{}^\circ $alcohol. In tert – butyl alcohol, the OH group is attached with three carbon atoms, so it is tertiary $3{}^\circ $alcohol.
These $1{}^\circ ,2{}^\circ ,3{}^\circ $alcohols are distinguished by Lucas reagent test. Lucas reagent is anhydrous zinc chloride and hydrochloric acid in the ratio 1:1 $ZnC{{l}_{2}}+HCl$. By reacting with Lucas reagent, alcohols form halides (alkyl chlorides) which have variable solubility. So, on reaction,
n – butyl alcohol is a primary alcohol, so it will have no reaction with Lucas reagent as:
$C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow{ZnC{{l}_{2}}+HCl}no\,reaction$
Sec – butyl alcohol, will form a turbid solution after 5 minutes due to the formation of secondary halide as:

Tert – butyl alcohol will show immediate turbidity in the reaction as it is a tertiary halide as:

Hence, n – Butyl, sec – butyl and tert – Butyl alcohols can be distinguished by the turbidity formed on reaction with Lucas reagent that is $ZnC{{l}_{2}}+HCl$.
Note:
The decreasing order of the reaction of alcohols towards Lucas reagent is$3{}^\circ >2{}^\circ >1{}^\circ $ as tertiary alcohols will give immediate turbidity with Lucas reagent. This test is based on the fact that alcohols are soluble in Lucas reagent, but the halides are not soluble, so they will form a turbid solution with cloudiness.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




