
Dipole moment of which ketone is maximum?
(A)
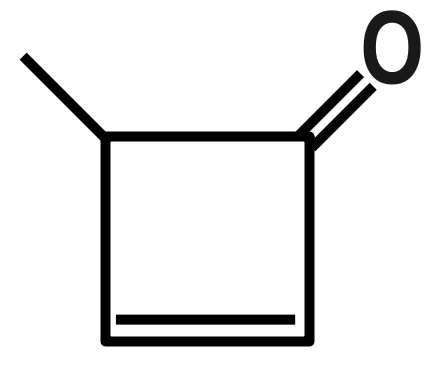
(B)
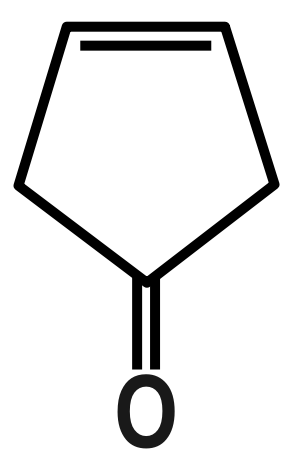
(C)
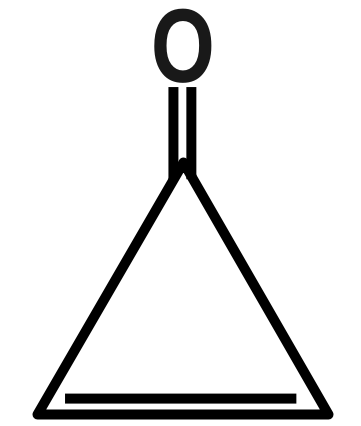
(D)
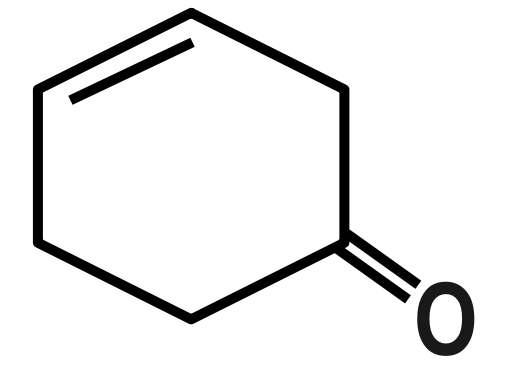




Answer
528.3k+ views
Hint: We know that for calculating the dipole moment we have to consider the magnitude and distance between the charges, but for estimating the highest and lowest dipole moment we can easily find them. If the bonds of carbon are symmetrical that are attached with the same atom it has zero dipole moment but when there is substitution then there is change in dipole moment.
Complete step by step solution:
We know that bonds can be classified as polar and nonpolar because of the electronegativity difference so that polarity is measured in terms of what we call dipole moment. It is defined as the product of magnitude of charge and distance between the charges; mathematically we can write it as
$ Dipole\,Moment(\mu )=q\times d. $
Here, is the charge and is the distance between the charges. Now in the above example we have some organic compounds so try to understand their structure as a sphere. So our option is benzene which is symmetrical therefore no dipole moment is there. In the second option we have naphthalene which can be considered as two benzene rings are attached so that’s why we can also see it as spherical and symmetrical.
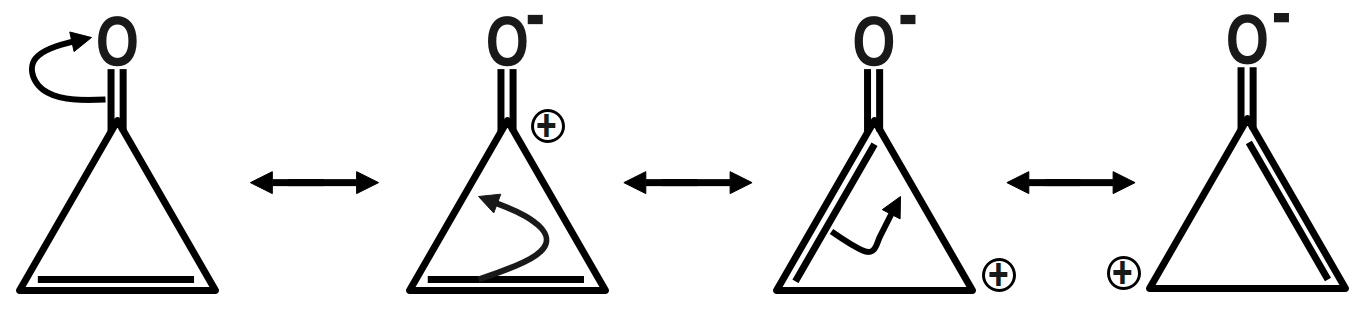
Therefore, the correct answer is option C.
Note:
Remember that Dipole moment has a unit of Debye, which is represented by capital (D). Now there are many examples in which we have to compare between the examples by the only difference we see. See as in options B and D we have a benzophenone type of system but in option B there is little bit difference in the structure by introduction of three membered rings that’s why its dipole moment is greater than benzophenone.
Complete step by step solution:
We know that bonds can be classified as polar and nonpolar because of the electronegativity difference so that polarity is measured in terms of what we call dipole moment. It is defined as the product of magnitude of charge and distance between the charges; mathematically we can write it as
$ Dipole\,Moment(\mu )=q\times d. $
Here, is the charge and is the distance between the charges. Now in the above example we have some organic compounds so try to understand their structure as a sphere. So our option is benzene which is symmetrical therefore no dipole moment is there. In the second option we have naphthalene which can be considered as two benzene rings are attached so that’s why we can also see it as spherical and symmetrical.

Therefore, the correct answer is option C.
Note:
Remember that Dipole moment has a unit of Debye, which is represented by capital (D). Now there are many examples in which we have to compare between the examples by the only difference we see. See as in options B and D we have a benzophenone type of system but in option B there is little bit difference in the structure by introduction of three membered rings that’s why its dipole moment is greater than benzophenone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




