
Why dipole moment of \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] is zero but that of \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] is non-zero?
Answer
589.8k+ views
Hint: \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] and \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] are the type of phosphorous Penta halide. The hybridization of these compounds is \[{\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}\]. And the geometry is trigonal bipyramidal. Where the less electronegative atoms are in equatorial position and more electronegative atoms are in axial position.
Complete step by step answer:
Now in the structures of \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] and \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] the axial positions are occupied by two fluorine atoms where the dipole moments of each \[{\text{P - F}}\] bonds nullify with each other. In case of equatorial bonds of \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] all three \[{\text{P - Cl}}\] bond dipoles nullify with each other. Therefore in \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] all the bond dipoles get cancel out and shows zero dipole moment.
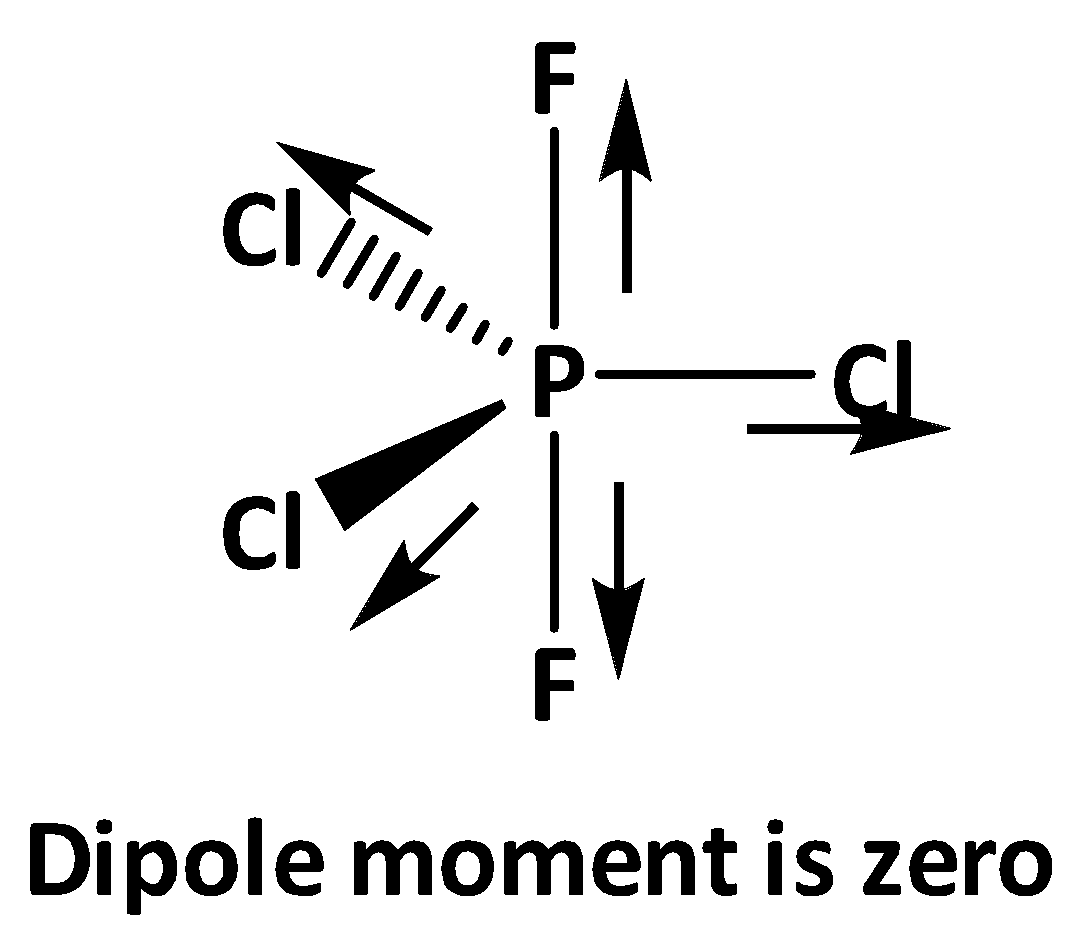
On the other hand, for \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] in the equatorial bonds two chlorine and one fluorine is present, as electronegativity of fluorine is greater than chlorine , the overall net dipole moment is to the direction of the \[{\text{P - F}}\] equatorial bond.
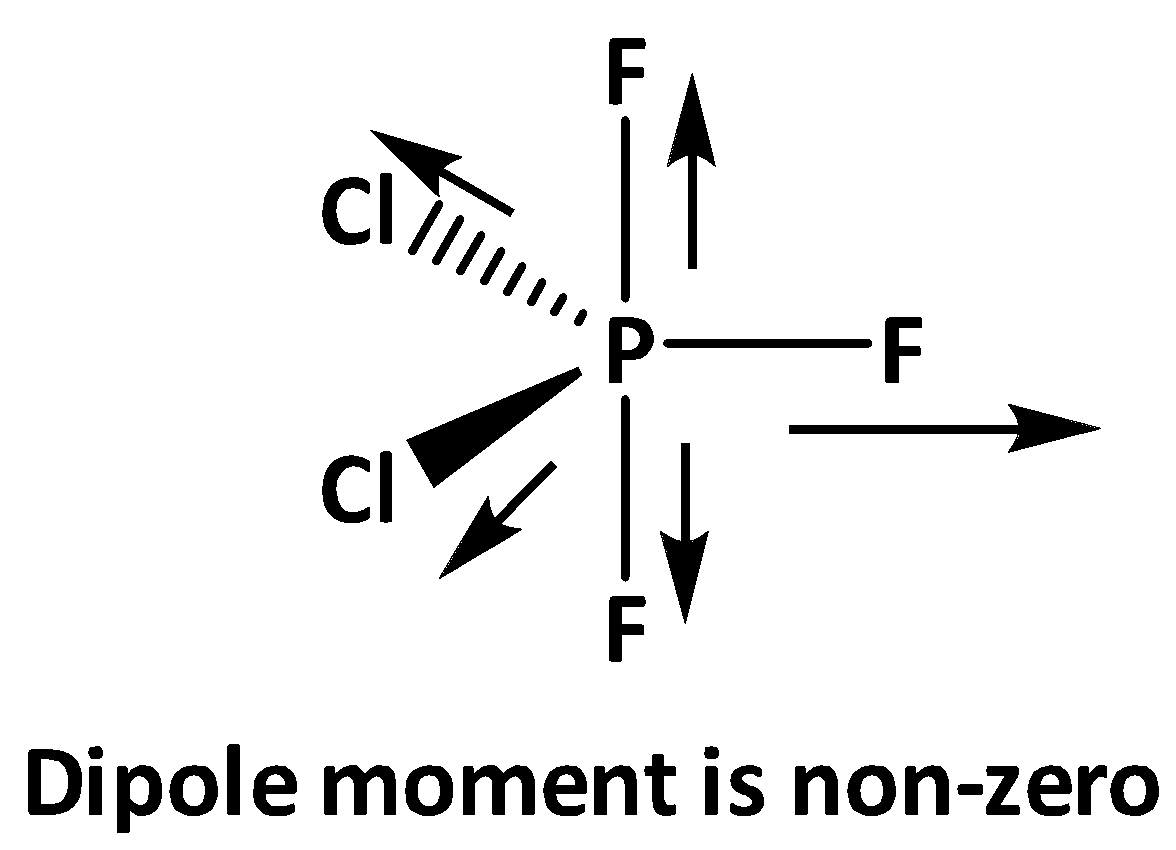
That is why \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] shows a non-zero dipole moment. On the other hand, \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\]has no dipole moment.
Note:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes) suitable for pairing of electrons to form chemical bonds .Hybrid orbitals are the combination of standard atomic orbitals resulting in the formation of new atomic orbitals.
Complete step by step answer:
Now in the structures of \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] and \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] the axial positions are occupied by two fluorine atoms where the dipole moments of each \[{\text{P - F}}\] bonds nullify with each other. In case of equatorial bonds of \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] all three \[{\text{P - Cl}}\] bond dipoles nullify with each other. Therefore in \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] all the bond dipoles get cancel out and shows zero dipole moment.

On the other hand, for \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] in the equatorial bonds two chlorine and one fluorine is present, as electronegativity of fluorine is greater than chlorine , the overall net dipole moment is to the direction of the \[{\text{P - F}}\] equatorial bond.

That is why \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\] shows a non-zero dipole moment. On the other hand, \[{\text{P}}{{\text{F}}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{2}}}\]has no dipole moment.
Note:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes) suitable for pairing of electrons to form chemical bonds .Hybrid orbitals are the combination of standard atomic orbitals resulting in the formation of new atomic orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




