
Why is dimethylformamide a polar aprotic solvent?
Answer
494.1k+ views
Hint: A polar aprotic solvents are those solvents which lack hydrogen atoms and are polar in nature. Such types of solvents have fewer hydroxyl and amine groups. They do not behave like proton donors in hydrogen bonding, even though they can be proton acceptors.
Complete Step By Step Answer:
As we know that various solvents are used in the chemical reactions and hence we should choose them wisely. A solvent is a substance in which solute dissolves to form a solution. The quantity of solute that dissolves in a specific volume of solvent varies with respect to temperature. There are two ways in which solvent can be used. One of the uses of the solvent is to behave as a medium where it does not take part in the reaction but eases the reaction by dissolving the solute in it and the other is to participate in the reaction by providing an acidic or basic source of the electrons.
The solvents are categorized into two parts namely polar and nonpolar. As by name we can suggest, in polar solvents we found polarity because of bond dipole moment whereas in nonpolar solvents there is no polarity because of absence of bond dipole moment. Further, polar solvents are divided into protic and aprotic which depend upon the availability of protons. If it contains proton then it is protic solvent whereas if it does not contain proton then it is aprotic solvent.
So, as we can see in the given structure:
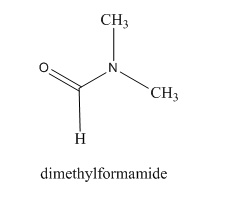
The polar $ C = O $ and $ C - N $ bonds make the whole molecule polar. But there are no $ O - H $ or $ N - H $ bonds hence the molecule is aprotic. Thus, dimethylformamide is an aprotic solvent.
Note:
We should remember that aprotic solvent can contain hydrogen atoms in the molecules but these hydrogen atoms are not attached to electronegative atoms such as $ O $ , $ N $ etc. directly. Hence because of the absence of $ O - H $ , $ N - H $ bonds the molecule is aprotic.
Complete Step By Step Answer:
As we know that various solvents are used in the chemical reactions and hence we should choose them wisely. A solvent is a substance in which solute dissolves to form a solution. The quantity of solute that dissolves in a specific volume of solvent varies with respect to temperature. There are two ways in which solvent can be used. One of the uses of the solvent is to behave as a medium where it does not take part in the reaction but eases the reaction by dissolving the solute in it and the other is to participate in the reaction by providing an acidic or basic source of the electrons.
The solvents are categorized into two parts namely polar and nonpolar. As by name we can suggest, in polar solvents we found polarity because of bond dipole moment whereas in nonpolar solvents there is no polarity because of absence of bond dipole moment. Further, polar solvents are divided into protic and aprotic which depend upon the availability of protons. If it contains proton then it is protic solvent whereas if it does not contain proton then it is aprotic solvent.
So, as we can see in the given structure:

The polar $ C = O $ and $ C - N $ bonds make the whole molecule polar. But there are no $ O - H $ or $ N - H $ bonds hence the molecule is aprotic. Thus, dimethylformamide is an aprotic solvent.
Note:
We should remember that aprotic solvent can contain hydrogen atoms in the molecules but these hydrogen atoms are not attached to electronegative atoms such as $ O $ , $ N $ etc. directly. Hence because of the absence of $ O - H $ , $ N - H $ bonds the molecule is aprotic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




