
Dilute acetylene is passed through dil.\[{{H}_{2}}S{{O}_{4}}\] in the presence of\[HgS{{O}_{4}}\], the compound formed is
A.Ethanol
B.Acetone
C.Acetic acid
D.Ethanal
Answer
590.4k+ views
Hint: Alkynes react with water in presence of dil.\[{{H}_{2}}S{{O}_{4}}\] and \[HgS{{O}_{4}}\] forms aldehyde or ketone. The metal catalyst \[HgS{{O}_{4}}\]acts as a catalyst. This reaction is called the Kucherov reaction. Alkynes are going to convert into aldehydes or ketones.
Complete answer:
The Kucherov reaction is an example of a hydration reaction. For hydration of Alkenes strong acid is sufficient.
Alkynes are less reactive towards hydration so addition of mercury sulfates benefits to catalyze the hydration reaction.
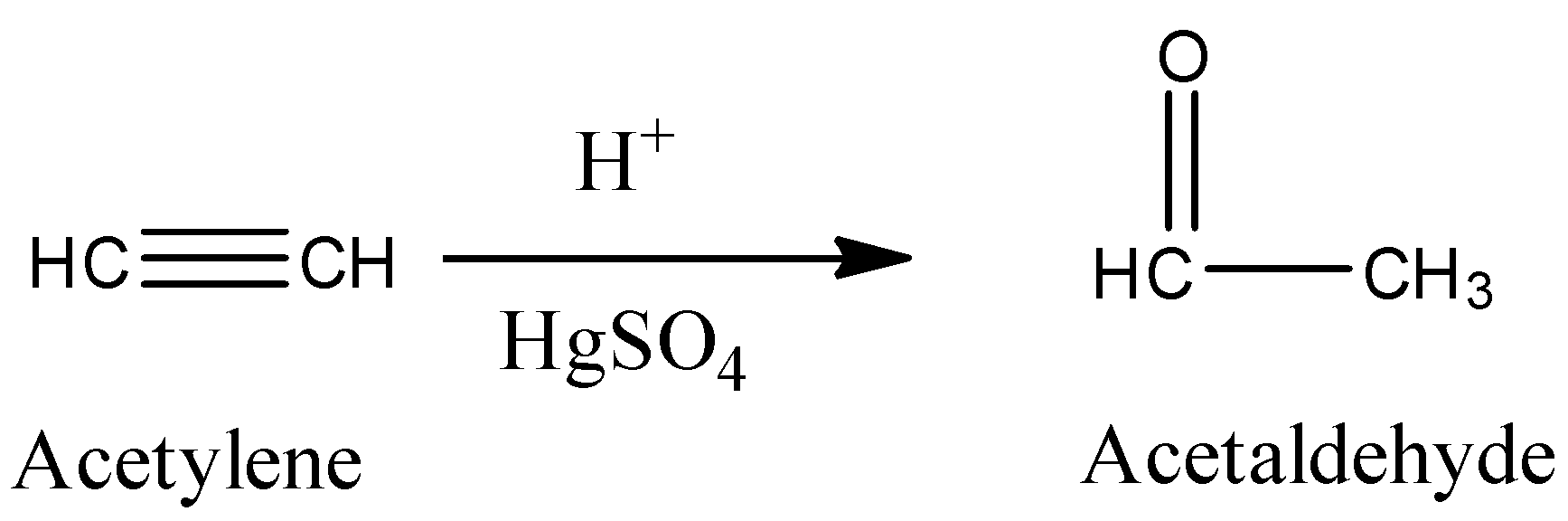
Hydration of acetylene in the presence of dil.\[{{H}_{2}}S{{O}_{4}}\], \[HgS{{O}_{4}}\]give acetaldehyde as a product.
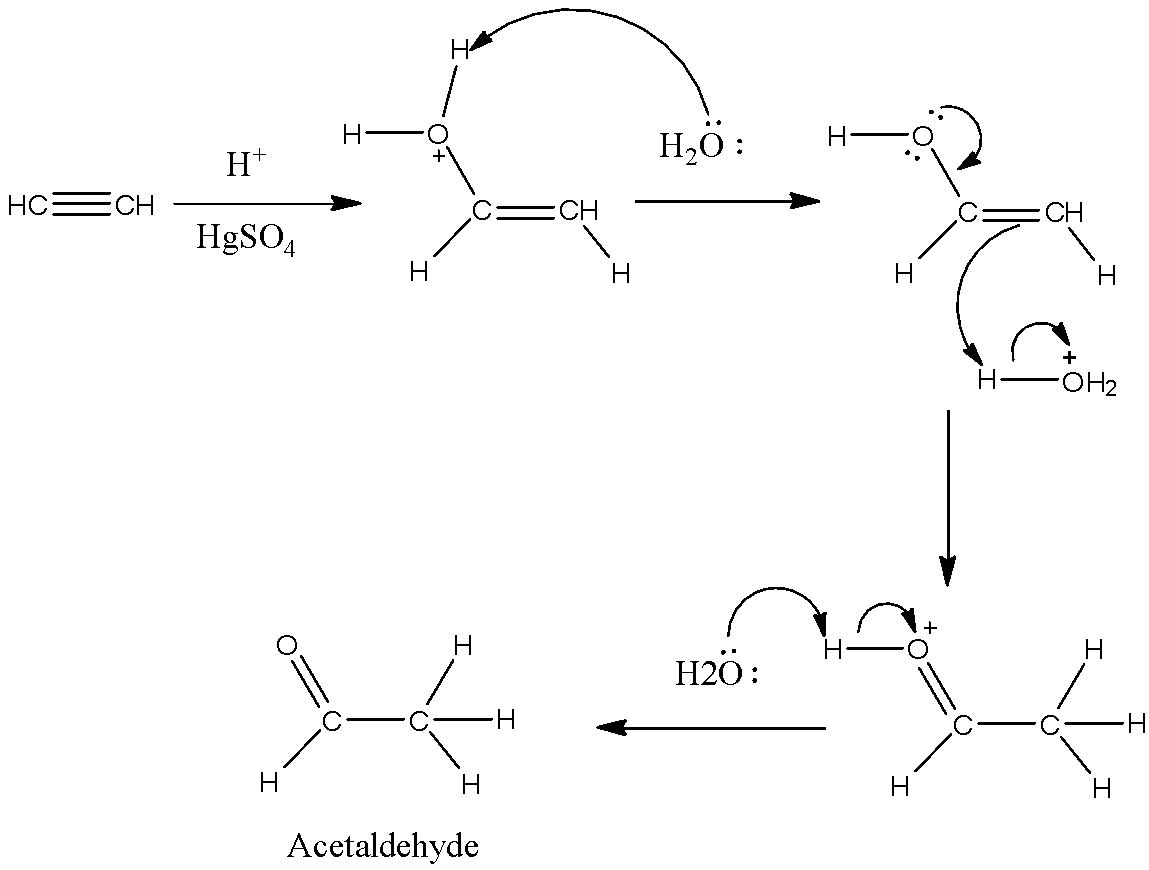
The mechanism of Kucherov reaction is as follows.
It is better to take symmetrical or terminal alkynes in the Kucherov reaction, otherwise formation of a mixture of compounds may form as a product.
The product formed in the above reaction is acetaldehyde.
Acetaldehyde is also called Ethanal.
So, the correct option is D.
Additional information:
Acetaldehyde is used as a precursor to prepare acetic acid.
Acetaldehyde is used in the manufacturing of resins, vinegar and polyvinyl acetate.
It is used in the manufacturing of disinfectants, and perfumes.
Acetaldehyde used as a preservative for fruits and fishes.
Note:
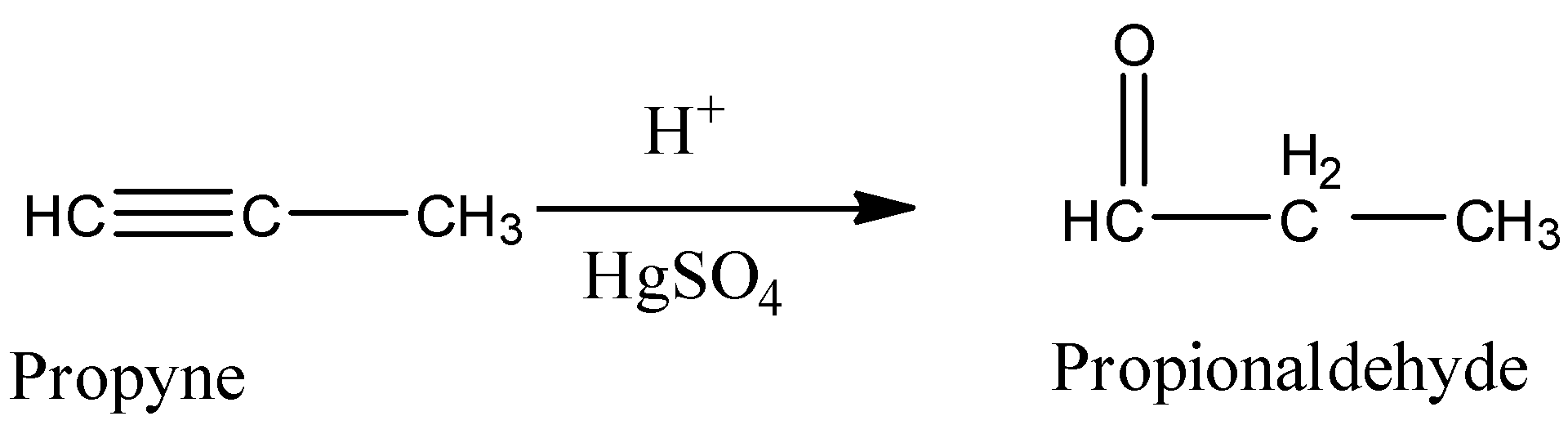
If we are going to take propyne or methyl acetylene as a precursor in Kucherov reaction then the resultant product is propionaldehyde or propanal. If the alkynes are highly substituted then we will get ketone as the product.
Complete answer:
The Kucherov reaction is an example of a hydration reaction. For hydration of Alkenes strong acid is sufficient.
Alkynes are less reactive towards hydration so addition of mercury sulfates benefits to catalyze the hydration reaction.
Hydration of acetylene in the presence of dil.\[{{H}_{2}}S{{O}_{4}}\], \[HgS{{O}_{4}}\]give acetaldehyde as a product.

The mechanism of Kucherov reaction is as follows.

It is better to take symmetrical or terminal alkynes in the Kucherov reaction, otherwise formation of a mixture of compounds may form as a product.
The product formed in the above reaction is acetaldehyde.
Acetaldehyde is also called Ethanal.
So, the correct option is D.
Additional information:
Acetaldehyde is used as a precursor to prepare acetic acid.
Acetaldehyde is used in the manufacturing of resins, vinegar and polyvinyl acetate.
It is used in the manufacturing of disinfectants, and perfumes.
Acetaldehyde used as a preservative for fruits and fishes.
Note:
If we are going to take propyne or methyl acetylene as a precursor in Kucherov reaction then the resultant product is propionaldehyde or propanal. If the alkynes are highly substituted then we will get ketone as the product.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




